THP1-Dual™ MD2-CD14-TLR4 Cells
-
Cat.code:
thpd-mctlr4
- Documents
ABOUT
CD14 and MD-2 expressing dual reporter monocytes for LPS studies
InvivoGen expands its collection of human monocytic reporter cell lines designed to monitor the TLR4 (Toll-like receptor 4)-dependent NF-kB and IRF responses upon stimulation with rough and smooth LPS (lipopolysaccharide):
— THP1-Dual™ MD2-CD14-TLR4 cells
— THP1-Dual™ MD2-CD14 KO-TLR4 (control) cells
These cells stably express MD-2 (myeloid differentiation factor 2) and CD14 (cluster of differentiation 14), two co-adaptors important for LPS-induced TLR4 signaling. Additionally, THP1-Dual™ MD2-CD14-TLR4 cells feature TLR4 overexpression and their control THP1-Dual™ MD2-CD14 KO-TLR4, a biallelic TLR4 knockout (KO). They were generated from the THP1-Dual™ cell line harboring two inducible reporter genes for SEAP (secreted embryonic alkaline phosphatase) and Lucia luciferase. This allows the simultaneous monitoring of the MyD88-dependent NF-κB and TRIF-dependent IRF pathway, respectively.
Due to the stable expression of CD14 and MD-2, the TRIF-dependent IRF response to LPS is completely restored in these cells. The MyD88-dependent NF-kB activation is strongly increased upon stimulation using smooth or rough LPS. The additional overexpression of hTLR4 enhances the sensitivity to various TLR4 ligands. As expected, the control cells THP1-Dual™ MD2-CD14 KO-TLR4 do not respond to any type of LPS (see figures below). Moreover, this KO cell line can be used to distinguish between TLR4- and other TLRs-mediated responses.
THP1-Dual™ MD2-CD14-TLR4 and their control derive from the human leukemia monocytic THP-1 cells. This cell line has become a common model to study monocyte/macrophage functions, mechanisms and signaling pathways. It expresses several pattern recognition receptors (PRRs), including TLR4, which upon sensing of their cognate PAMPs (pathogen-associated molecular patterns) trigger the activation of the NF-kB and/or IRF transcription factors [1]. TLR4-induced IRF-activation in THP-1-derived cells (e.g. THP1-Dual™) requires their differentiation into macrophages using PMA (Phorbol 12-myristate 13-acetate). Overexpression of MD-2/CD14 in THP1-Dual™ MD2-CD14-TLR4 and THP1-Dual™ MD2-CD14 KO-TLR4 (control) cells eliminates this requirement, thus TLR4-dependent IRF activation can be studied without PMA treatment.
The adaptor proteins, MD-2 and CD14, are essential for TLR4-dependent sensing of rough or smooth LPS. The wildtype, smooth (s)LPS requires CD14 co-expression, whereas the truncated form of LPS (rough LPS) can also interact with TLR4 in a CD14-independent manner. Their recognition lead to NF-κB and IRF activation and the production of proinflammatory cytokines and interferons, respectively [2-3]. Thus, the LPS-induced TLR4 responses are highly inflammatory, which makes it a remarkable therapeutic target [3].
![]() Bypass PMA Differentiation Bias: THP1-Dual™ MD2-CD14-TLR4 cells (& control) do not require PMA treatment prior to IRF-stimulation (see figures).
Bypass PMA Differentiation Bias: THP1-Dual™ MD2-CD14-TLR4 cells (& control) do not require PMA treatment prior to IRF-stimulation (see figures).
Key Features
- Stable overexpression of CD14 and MD-2
- Verified overexpression or biallelic KO of the hTLR4 gene
- Increased and reproducible response to rLPS and sLPS
- Measurable IRF response without PMA-differentiation
- Distinct monitoring of NF-κB or IRF activation by assessing the SEAP and Lucia luciferase activities
Applications
- Detecting sample contamination with rLPS and sLPS
- Defining the role of TLR4 in LPS-induced signaling pathways
- Screening for TLR4 agonists or antagonists
- Highlighting possible overlap between TLR4 and other TLR-related signaling pathways (e.g. TLR2)
References:
1. Hornung V. et al. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol.; 168(9):4531-7.
2. Cochet, F. et al. 2017. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int J Mol Sci 18.
3. Kuzmich, N.N. et al. 2017. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel) 5.
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
TLR4
Human
Dual reporter assays for TLR4 pathway, NF-κB and IRF signaling pathways
Complete RPMI 1640 (see TDS)
Verified using Plasmotest™
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:THP1-Dual™ MD2-CD14-TLR4 Cells
-
Cat code:thpd-mctlr4
-
Quantity:3-7 x 10^6 cells
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Blasticidin (10 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
Storage:
Details
Types of LPS
Lipopolysaccharide (LPS) is a major constituent of the outer membrane of Gram-negative bacteria. It comprises three covalently linked regions:
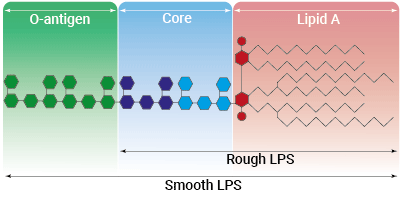
- the lipid A (endotoxin)
- the rough core oligosaccharide
- the O-antigenic side chain.
Wild-type LPS contains the O-side chain and is referred to as smooth (sLPS). Few bacterial strains (e.g. Salmonella Thyphimurium, Brucella canis) lost the O-side chain. This mutated form is called rough (rLPS). Both sLPS and rLPS share the same receptor complex (TLR4-MD-2-CD14), but their mechanism of action differs. While CD14 is necessary for sLPS NF-κB and IRF signaling, it is dispensible for rLPS NF-κB signaling. It has been hypothesized that rLPS activates a broader range of cells (CD14 positive, low and negative), accounting for higher toxicity [1].
Nevertheless, both LPS variations elicit potent innate immune responses. The resulting signaling triggers the release of pro‑inflammatory cytokines, which can lead to both acute and chronic inflammatory diseases. It is all about balance: small controlled amounts of LPS can be protective and large uncontrolled amounts can lead to disastrous outcomes, such as septic shock [2]. Contamination by LPS is a major threat for health, research and industry, as it can interfere with signaling cascades and cytokine production or induce septic shock in vivo [3]. Thus, its accurate detection as well as the discrimination between LPS-induced and non-LPS-induced signaling is crucial to obtain unbiased results and 'sterlie' products. However, despite its highly inflammatory nature, LPS has remarkable therapeutic potential and features many characteristics needed for an effective vaccine adjuvant [4].
Toll-like receptor 4 signaling
The Toll-like receptor 4 (TLR4) was the first TLR identified and is an important pattern recognition receptor (PRR) in innate immunity and inflammation. It is found both on the cell surface and in endosomes of innate immune cells including monocytes and macrophages, as well as on intestinal epithelium and endothelial cells [5]. TLR4 can recognize pathogen- and damage-associated molecular patterns (PAMPs and DAMPs). However, it is primarily activated by lipopolysaccharide (LPS) and its toxic moiety Lipid A [6]. TLR4 does not directly interact with LPS, but requires essential adaptor proteins [3]. The soluble LPS-binding protein (LBP) extracts monomeric LPS from the microbial membrane and transfers it to CD14 (cluster of differentiation 14). This membrane-bound protein then interacts with MD-2 (myeloid differentiation factor 2), which is constitutively associated with the TLR4 ectodomain. The ligand-loaded MD-2 subsequently binds to another TLR4/MD-2/LPS complex, leading to their dimerization [7]. Then, TLR4 triggers two distinct signaling cascades [5]:
- the MyD88-dependent activating NF-κB pathway (at the cell surface)
- the TRIF-dependent activating IRF pathway (in endosomes)
At the cell surface, activation of TLR4 initiates the TIRAP-MyD88-dependent pathway, ultimately leading to the activation of NF-κB and the production of a pro-inflammatory response. Also, the TLR4 complex can be endocytosed into endosomes in a CD14-mediated fashion. This results in the the stimulation of IRF3 (interferon regulatory factor), which modulates the expression of type I IFN [3].
TLR4 signaling is crucial in both acute and chronic inflammatory disorders and thus, is an attractive target for novel treatments [5]. Stimulating drugs are useful for the development of vaccine adjuvants or cancer immunotherapeutics, whereas TLR4-inhibition is a therapeutic approach to treat septic shock or autoimmune inflammatory pathologies such as atherosclerosis [8].
References:
1. Zanoni I,.et al., 2012. Similarities and differences of innate immune responses elicited by smooth and rough LPS. Immunol Lett. 2012 Feb 29;142(1-2):41-7.
2. Godowski, P., 2005. A smooth operator for LPS responses. Nat Immunol 6, 544–546.
3. Kuzmich, N.N. et al. 2017. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel) 5.
4. McAleer, J.P. & Vella, A.T., 2010. Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol 31, 429-435.
5. Ou, T. et al. 2018. The Pathologic Role of Toll-Like Receptor 4 in Prostate Cancer. Front Immunol 9, 1188.
6. Cochet, F. et al. 2017. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int J Mol Sci 18
7.Tanimura N. et al. 2014. The attenuated inflammation of MPL is due to the lack of CD14-dependent tight dimerization of the TLR4/MD2 complex at the plasma membrane. Int Immunol.(6):307-14.
8. Romerio A, Peri F. 2020. Increasing the Chemical Variety of Small-Molecule-Based TLR4 Modulators: An Overview. Front Immunol.;11:1210.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?












