Anti-hIL-9-hIgG1
-
Cat.code:
hil9-mab1NEW
- Documents
ABOUT
Anti-human IL-9 - Enokizumab biosimilar - CAS #909875-08-7
Anti-hIL-9-hIgG1 is a biosimilar antibody of Enokizumab, a human interleukin 9 (IL-9) antibody that inhibits IL-9 signaling. This monoclonal antibody (mAb) is currently under investigation for the treatment of asthma, atopic dermatitis, and inflammatory bowel diseases (IBD).
Anti-hIL-9-hIgG1 comprises the variable region of Enokizumab and the IgG1 constant region of Enokizumab for high effector functions.
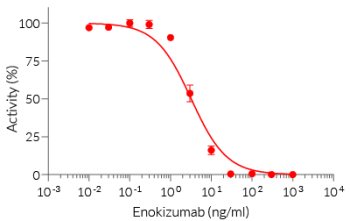
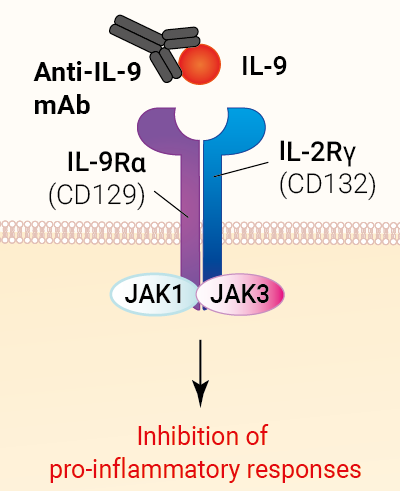
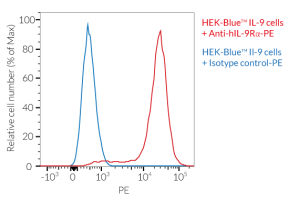
This mAb can be used together with HEK-Blue™ IL-9 cells for screening and neutralization assays to inhibit IL-9 signaling induced by recombinant human IL-9 (see figure).
Key features
- Each lot is functionally tested and validated.
- The complete sequence of the antibody construct was verified.
- The absence of endotoxins is determined using the EndotoxDetect™ assay.
All products are for research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
IL-9
Human
Sodium phosphate buffer, glycine, saccharose, stabilizing agents
Negative (tested using EndotoxDetect™ assay)
Neutralization assay, ELISA
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Anti-hIL-9-hIgG1
-
Cat code:hil9-mab1
-
Quantity:100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20 °C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Enokizumab and IL-9 background
Enokizumab (MEDI-528) is a humanized monoclonal antibody (mAb) designed to target interleukin 9 (IL-9) and block the IL-9 signaling [1].
IL-9 is a cytokine produced by a wide variety of cells including mast cells, natural killer T (NKT) cells, Th2, Th17, Treg, and Th9 cells. Th9 cells are considered to be the main CD4+ T cells that produce IL-9. To date, its main role has been found in the immune responses against allergic diseases such as asthma and bronchial hyperreactivity. IL-9 promotes the survival, proliferation, and activation of mast cells and other immune cells, which are central to the development of allergic reactions and inflammatory diseases [2].
Enokizumab is currently being investigated for its potential therapeutic applications in various inflammatory and allergic diseases, with a particular focus on asthma, atopic dermatitis, and inflammatory bowel diseases (IBD). In asthma treatment, it has shown promise in patients with a specific IL-9-driven inflammatory profile [2-3]. Asthma, particularly in severe forms, is characterized by airway inflammation and hyperreactivity, often triggered by immune cells and cytokines like IL-9 [2-3]. However, despite the encouraging data from some trials, regulatory approval for the drug is still pending.
References:
1. Parker JM, et al., 2011. Safety profile and clinical activity of multiple subcutaneous doses of MEDI-528, a humanized anti-interleukin-9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulm Med. 11:14.
2. Bick F, et al., 2025. A reappraisal of IL-9 in inflammation and cancer. Mucosal Immunol. (1):1-15.
3. NCT00590720: a Phase 2A Study to Evaluate the Safety and Effect on Exercise Challenge Testing of MEDI-528 in Adults With Asthma.
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Validation Data Sheet
Certificate of analysis
Need a CoA ?