THP1-Dual™ hTLR3 Cells
-
Cat.code:
thpd-htlr3
- Documents
ABOUT
Dual reporter monocytes for TLR3 pathway studies
InvivoGen offers a human monocyte-derived cell line, specifically designed for the study of human TLR3 (Toll-Like Receptor 3)-dependent signaling pathways:
— THP1-Dual™ hTLR3 cells
These cells were generated from the THP1-Dual™ cell line harboring two inducible reporter genes, allowing the simultaneous study of the NF-κB and IRF pathway, by monitoring the SEAP and Lucia luciferase activities, respectively. They overexpress human TLR3 as well as important elements for its signaling upon double-stranded (ds)RNA stimulation.
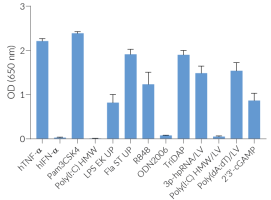
THP1-Dual™ hTLR3 cells are responsive to synthetic analogs of dsRNA, in contrast to their parental cell line THP1-Dual™ (see figures). They show potent NF-κB and IRF responses upon incubation with TLR3-specific ligands, such as Poly(I:C) (polyinosinic-polycytidylic acids) or Poly(A:U) (polyadenylic–polyuridylic acid).
Of note, as THP-1 cells express endogenous levels of various TLRs [1] and THP1-Dual™ -derived cells respond to their cognate ligands (e.g. Pam3CSK4, LPS, flagellin) (see figures).
Upon recognition of viral or synthetic dsRNA, TLR3 mediates the transcriptional induction of type I interferons (IFNs), proinflammatory cytokines, and chemokines, thereby collectively establishing an antiviral host response [2].
Key features:
- Stable overexpression of human TLR3 and important elements facilitating its signaling
- Restored response to dsRNA and analogs
- Distinct monitoring of TLR3-dependent NF-κB or IRF activation by assessing the SEAP and Lucia® luciferase activities
Applications:
- Defining the role of TLR3-dependent IRF and NF-κB signaling pathways
- Screening for novel TLR3 agonists and inhibitors
References
1. Hornung V. et al. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol.; 168(9):4531-7.
2. Chen Y, et al., 2021. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J Zhejiang Univ Sci B.;22(8):609-632.
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
TLR3 activation cellular assay
Complete RPMI 1640 (See TDS)
Verified using Plasmotest™
Each lot is functionally tested and validated
CONTENTS
Contents
-
Product:THP1-Dual™ hTLR3 Cells
-
Cat code:thpd-htlr3
-
Quantity:3-7 x 10^6 cells
1 ml of Blasticidin (10 mg/ml) 1 ml of Zeocin® (100 mg/ml) 1 ml of Normocin™ (50 mg/ml). Normocin™ 1 ml of QB reagent and 1 ml of QB buffer 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent
Shipping & Storage
- Shipping method: Dry ice
- Liquid Nitrogen Vapor
- Upon receipt, store immediately in liquid nitrogen vapor. Do not store cell vials at -80°C.
Storage:
Caution:
Details
Toll-Like Receptor 3
In humans, four Toll-Like Receptor (TLR) family members TLR3, TLR7, TLR8, and TLR9 are specialized in sensing viral-derived components and are mainly found in the endosome. Among these, TLR3 recognizes double-stranded (ds)RNA, a hallmark of viral replication, and triggers antiviral immune responses [1]. TLR3 is expressed in myeloid dendritic cells, macrophages, as well as non-immune cells [2].
TLR3 signaling
TLR3 activation upon viral infection involves several steps, including translocation of TLR3 from the ER (endoplasmic reticulum) via the Golgi to the endosome, proteolytic cleavage and dimerization of TLR3, and finally receptor-ligand binding [3]. In order to start the signaling cascade, activated TLR3 recruits the adaptor protein TRIF (TIR domain-containing adapter-inducing interferon-β). TRIF binds to TRAF3 (TNF receptor-associated factor 3), which then recruits TBK1 (TANK-binding kinase 1) and IKKε (IκB kinase ε), thus activating the transcription factor IRF3 (interferon regulatory factor 3) and stimulating the production of type I IFNs (interferons). Additionally, TRIF interacts with TRAF6 and RIP1 (kinase receptor-interacting protein 1). RIP1 in turn binds to TAK1 (transforming growth factor β-activated kinase 1) and IKK. TAK1 phosphorylates IKKα and IKKβ, leading to the phosphorylation of IκB, the NF-κB inhibitor. Ultimately, this leads to the release and translocation of NF-κB into the nucleus and the induction of pro-inflammatory cytokines [2,4].
Pathology
Given its important role in dsRNA recognition, TLR3 signaling has been intensively studied. Various TLR3-agonists, such as the synthetic dsRNA analog Poly(I:C) are being used in vaccine development and cancer therapy [4]. Yet, recent studies have indicated that TLR3 may act as a double-edged sword by showing both protective and damaging functions in the context of some human viral infections [3,5]. Moreover, rare mutations in TLR3 have been associated with viral susceptibility; specifically, infections with HSV-1 (herpes simplex virus 1), influenza, and SARS-Co-V2 have been linked to pathogenic germline variants in TLR3 pathway genes [2]. Understanding the TRIF-dependent TLR3 pathway may be essential for the establishment of specific therapeutic approaches to diminish TLR3-driven disease and exploit its protective functions [3].
References
1. Manuela Sironi, et al., 2012. A Common Polymorphism in TLR3 Confers Natural Resistance to HIV-1 Infection. J Immunol 15; 188 (2): 818–823.
2. Aluri, J, et al., 2021. Toll-Like Receptor Signaling in the Establishment and Function of the Immune System. Cells, 10, 1374.
3. Chen Y, et al., 2021. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J Zhejiang Univ Sci B.;22(8):609-632.
4. Komal A, et al., 2021. TLR3 agonists: RGC100, ARNAX, and poly-IC: a comparative review. Immunol Res. 69(4):312-322.
5. Perales-Linares R, Navas-Martin S. 2013. Toll-like receptor 3 in viral pathogenesis: friend or foe? Immunology.;140(2):153-67.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?