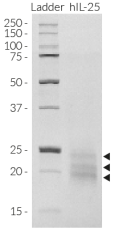
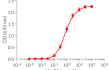
Recombinant human IL-25 (IL-17E)
-
Cat.code:
rcyc-hil25NEW
- Documents
ABOUT
Human IL-25 protein - Mammalian cell-expressed, tag-free, carrier-free
Recombinant human IL-25 (IL-17E) is a high-quality and biologically active cytokine, validated using proprietary IL-25 reporter cells. This pro-inflammatory cytokine is produced in HEK293 cells to ensure protein glycosylation and bona fide 3D structure.
Recombinant human IL-25 can be used together with HEK-Blue™ IL-25/IL-17C cells for the screening of inhibitory molecules, such as monoclonal antibodies targeting IL-25 or its receptor.
Key features
- Each lot is validated using HEK-Blue™ IL-25/IL-17C cells
- Endotoxin ≤ 0.1 EU/µg
- 0.22 µm sterile-filtered
Applications
- Standard for IL-25 detection and quantification
- Screening and release assays for antibodies blocking IL-25 signaling
- Screening and release assays for engineered IL-25
Interleukin-25 (also known as IL-17E) is a member of the IL-17 family (IL-17A – 17F). IL-25 is mainly produced by epithelial cells and promotes the expression of type 2 cytokines in barrier tissues (e.g., skin, lungs, gastrointestinal tract). Dysregulated IL-25 expression has been associated with multiple inflammatory disorders such as atopic dermatitis, psoriasis, or asthma.
All InvivoGen products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
Q9H293
100 μg/ml in water
Phosphate buffer saline (pH 7.0), glycine
0.22 µm filtration
The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK‑Blue™ TLR4 cells.
Cellular assays (tested)
ELISA
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Recombinant human IL-25 (IL-17E)
-
Cat code:rcyc-hil25
-
Quantity:20 µg
1.5 ml endotoxin-free water
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
IL-25 background
Interleukin 17 (IL-17) is a family of six closely related cytokines (IL-17A to IL-17F) that exhibit both pro- and anti-inflammatory activity. IL-25 (also known as IL-17E) is primarily described as a "barrier surface" cytokine. It is produced by various cell types, including T helper 2 (Th2) cells, mast cells, or epithelial cells, and it acts on both immune and tissue-resident cells in barrier tissues (e.g., skin, lungs, gastrointestinal tract) [1, 2].
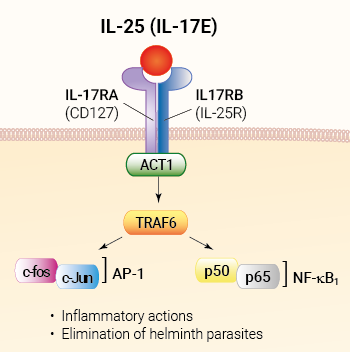
IL-25 binds a homodimeric transmembrane receptor that comprises the IL-17RA subunit shared by all IL-17 isoform receptors and the IL-17RB subunit. The binding of IL-25 to its receptor triggers the recruitment of ACT1 (activator of NF-κB1), which recruits and ubiquitinates TRAF6 (TNF receptor-associated factor 6). The subsequent signaling cascade leads to the activation of the canonical NF-κB and AP-1 pathways [1, 2]. While all IL-17 family members promote inflammatory neutrophilic responses, IL-25 induces the expression of type 2 cytokines (i.e., IL-4, IL-5, IL-13, and TSLP). This functional divergence led to the renaming of IL-17E as IL-25 [2].
Relevance for therapeutics development
Dysregulated IL-25 expression has been associated with multiple inflammatory disorders.
- In the skin, IL-25 contributes to epidermal homeostasis under steady state conditions [1]. However, elevated IL-25 expression is found in the lesions of skin disorders, such as atopic dermatitis, psoriasis, and contact dermatitis [1].
- In the gut, IL-25 expression by chemosensory cells is upregulated upon parasite infection. IL-25 then contributes to recruiting Th2 cells and ILC2 (type 2 innate lymphoid cells) to mediate anti-helminth immunity [1]. IL-25 has been reported to play both pathogenic and protective functions in different inflammatory bowel disease (IBD) settings, which is characterized by an imbalance of the Th1/Th2 cytokine response. Although extensive data have been generated in experimental colitis models, the precise role of IL-25 in ulcerative colitis patients remains incompletely defined [1].
- In the airways, IL-25 expression by chemosensory cells is induced in response to infections and allergens. While IL-25 may contribute to epithelial protection, sustained type-2 immune activation has been implicated in the pathogenesis of chronic rhinosinusitis and asthma [1].
Given its central role in type-2 immunity, therapeutic blockade of IL-25 signaling represents a promising strategy to alleviate Th2-driven inflammatory diseases. SM17, a humanized monoclonal antibody targeting the IL-17RB subunit of the IL-25 receptor, has completed a phase I clinical trial (NCT05332834), demonstrating a favorable safety, tolerability, and pharmacokinetic profile. Further clinical development is ongoing for the treatment of atopic dermatitis. In preclinical mouse models, administration of IL-25-blocking antibodies has been shown to ameliorate Th2-mediated airway inflammation [1].
References:
1. Borowczyk, J., et al., 2021. IL-25 (IL-17E) in epithelial immunology and pathophysiology. Journal of Allergy and Clinical Immunology. 148(1):40-52.
2. McGeachy, M.J. et al., 2019. The IL-17 Family of Cytokines in Health and Disease. Immunity. 50(4):892-906.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?