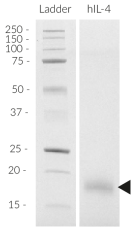
Recombinant human IL-4 (CHO)
-
Cat.code:
rcyc-hil4NEW
- Documents
ABOUT
Recombinant human IL-4 protein - Mammalian cell-expressed, tag-free, carrier-free
Recombinant human IL-4 is a high-quality and biologically active cytokine, validated using proprietary IL-4/IL-13 reporter cells. This member of the IL-2/γc superfamily is produced in CHO cells to ensure protein glycosylation and bona fide 3D structure.
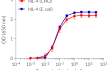
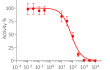
Recombinant human IL-4 can be used together with HEK-Blue™ IL-4/IL-13 cells for the screening of inhibitory molecules, such as Dupilumab, a therapeutic monoclonal antibody targeting the IL-4Rα subunit of the IL-4 receptor (see figures).
Key features
- Each lot is validated using HEK-Blue™ IL-4/IL-13 cells
- Endotoxin < 0.1 EU/µg
- 0.22 µm sterile-filtered
Applications
- Standard for IL-4 detection and quantification assays
- Screening and release assays for antibodies blocking IL-4 signaling
- Screening and release assays for engineered IL-4
Interleukin 4 (IL-4) shares a common receptor subunit, IL-4Rα, with IL-13. These two cytokines play an important role in anti-parasitic immune responses. Dysregulated IL-4 /IL-13 expression contributes to Th2-mediated diseases, including asthma and atopic dermatitis.
All products are for research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
P05112
100 μg/ml in water
Phosphate buffer saline (pH 7.4), saccharose
0.22 µm filtration
The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK‑Blue™ TLR4 cells.
Cellular assays
Cellular assays (tested)
ELISA
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Recombinant human IL-4 (CHO)
-
Cat code:rcyc-hil4
-
Quantity:20 µg
1.5 ml endotoxin-free water
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
IL-4 background
IL-4, also known as B cell stimulatory factor 1 or lymphocyte stimulatory factor 1, is a cytokine that belongs to the IL-2/γc superfamily. IL-4 is produced as a secreted monomeric molecule by activated CD4+ T cells, Natural Killer T cells (NKT cells), group 2 innate lymphoid cells (ILC2s), macrophages, mast cells, basophils, or eosinophils [1].
IL-4 binds two types of heterodimeric receptors and exerts pleiotropic actions on multiple cell lineages. The type I receptor is composed of the IL-4Rα and the common γ (cγ) subunits. The type II receptor is composed of the IL-4Rα and IL-13Rα1 subunits. The binding of IL-4 to its receptors triggers a signaling cascade leading to the activation of STAT6. Subsequent gene expression drives Th2 and Th9 helper cell differentiation and M2 macrophage polarization. In B cells, IL-4 promotes proliferation and IgE immunoglobulin class switching [1, 2]. In non-hematopoietic cells, the functions of IL-4 span from mucus hypersecretion in airway epithelial cells, extracellular matrix protein deposition by fibroblasts, and growth of endothelial cells [1]. Altogether, the downstream IL-4 effector functions participate in parasitic infection clearance and allergic reactions [2].
Relevance for therapeutics development
IL-4 is a key cytokine for controlling infections by extracellular parasites. However, along with IL-13, it also contributes to harmful allergic responses [2, 3]. As the prevalence of chronic allergic diseases such as asthma and atopic dermatitis is increasing worldwide, there has been a keen interest in the therapeutic blocking of IL-4 and IL-13 signaling.
Dupilumab is a fully human monoclonal antibody (mAb) that targets the IL-4Rα subunit [4]. It acts like a receptor antagonist and inhibits the signaling of both IL-4 and IL-13 [4]. Dupilimab was FDA-approved in 2017 for treating asthma, atopic dermatitis, and chronic sinusitis [4, 5]. Due to its favorable safety profile and established clinical use, Dupilumab holds significant promise for amending treatment options for many dermatologic conditions [6].
The development of engineered IL-4 cytokines has also been explored. For example, IL-4 "superkines" featuring a higher affinity for type I or type II receptors have been generated to target cells expressing lower second chain (γc or IL-13Rα1) numbers. To go further, engineered super-IL-4 fused to Pseudomonas toxin has been tested for targeted toxin delivery in tumor cells expressing IL-4 receptors [7].
References:
1. Keegan, A.D., et al., 2021. Recent advances in understanding the role of IL-4 signaling. Fac Rev. 10: 71.
2. Bernstein, Z.J., et al., 2023. Engineering the IL-4/IL-13 axis for targeted immune modulation. Immunol Rev. 320(1):29-57.
3. Ogulur, I., et al., 2025. Type 2 immunity in allergic diseases. Cell & Mol Immunol, 22(3):211-242.
4. Harb, H. & Chatila, T.A., 2020. Mechanisms of Dupilumab. Clin Exp Allergy. 50(1):5-14.
5. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761055s040lbl.pdf
6. Olbrich, H., et al., 2023. Dupilumab in Inflammatory Skin Diseases: A Systematic Review. Biomolecules.13(4):634.
7. Leonard, W.J & Lin, J.X, 2023. Strategies to therapeutically modulate cytokine action. Nat Rev Drug Discov. 22(10):827-854.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?