Anti-hIL-5R-hIgG1fut
-
Cat.code:
hil5r-mab13NEW
- Documents
ABOUT
Anti-human IL-5R - Benralizumab biosimilar - CAS #1044511-01-4
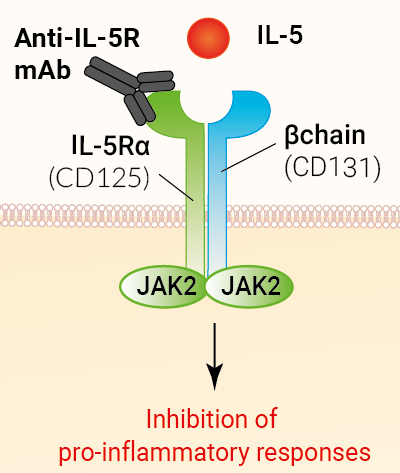
Anti-hIL-5R-hIgG1fut is a biosimilar antibody of Benralizumab, a human interleukin-5 receptor (IL-5R) antibody that blocks IL-5 signaling. This monoclonal antibody (mAb) specifically targets the alpha subunit of the IL-5R. Benralizumab, also known as MEDI-563, is FDA-approved for the treatment of severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis (EGPA).
Anti-hIL-5R-hIgG1fut comprises the variable region of Benralizumab and the afucosylated/non-fucosylated IgG1 constant region of Benralizumab for high effector functions.
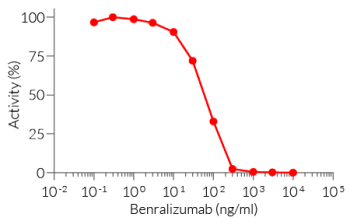
This mAb can be used together with HEK-Blue™ IL-5 cells for screening and neutralization assays to block IL-5 signaling induced by recombinant human IL-5 (see figure).
Key features
- Each lot is functionally tested and validated.
- The complete sequence of the antibody construct was verified.
- The absence of endotoxins is determined by the EndotoxDetect™ assay.
All products are for research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
IL-5R
Human
Neutralization assay (tested)
ELISA
Sodium phosphate buffer with glycine, saccharose, and stabilizing agents
Negative (tested using EndotoxDetect™ assay)
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Anti-hIL-5R-hIgG1fut
-
Cat code:hil5r-mab13
-
Quantity:100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Benralizumab is a humanized, afucosylated IgG1 monoclonal antibody (mAb) designed to target the alpha chain of the human interleukin 5 receptor (IL-5Rα), expressed on eosinophils and basophils [1]. IL-5 plays a critical role in the survival, proliferation, and activation of eosinophils, which contribute to airway inflammation and exacerbations in eosinophilic asthma [2]. In addition to inhibiting interleukin-5 signaling, Benralizumab leads to eosinophil apoptosis through ADCC [1]. Benralizumab is FDA-approved for the treatment of severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis (EGPA) [3].
References
1. Wechsler ME, et al., 2024. Benralizumab versus Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med. 390(10):911-921.
2. Kolbeck R, et al., 2010. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 125(6):1344-1353.e2.
3. Fasenra (benralizumab) US prescribing information; 2024. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761070s021lbl.pdf
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?