Anti-hIL-21-hIgG1
-
Cat.code:
hil21-mab1NEW
- Documents
ABOUT
Anti-human IL-21 - Avizakimab biosimilar - CAS #2229685-51-0
Anti-hIL-21-hIgG1 is a biosimilar antibody of Avizakimab, a human interleukin 21 (IL-21) antibody that blocks IL-21 signaling. This monoclonal antibody (mAb) has progressed to phase 1/2 clinical trials to treat patients with systemic lupus erythematosus (SLE).
Anti-hIL-21-hIgG1 comprises the variable region of Avizakimab and the IgG1 constant region of Avizakimab, mediating high effector functions.
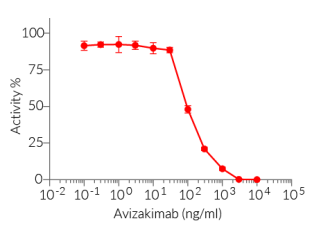
This antibody can be used together with HEK-Blue™ IL-21 cells for screening and neutralization assays to block signaling induced by recombinant human IL-21 (see figure).
Key features
- Each lot is functionally tested and validated.
- The complete sequence of the antibody construct was verified.
- The absence of endotoxins is determined by the EndotoxDetect™ assay.
All InvivoGen products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
IL-21
Human
Neutralization assay (tested), ELISA, ADCC
Sodium phosphate buffer, glycine, saccharose, stabilizing agents
0.2 µm filtration
Negative (tested using EndotoxDetect™ assay)
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Anti-hIL-21-hIgG1
-
Cat code:hil21-mab1
-
Quantity:100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Avizakimab background
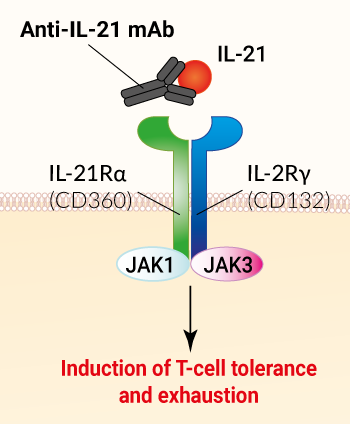
Avizakimab (aka BOS-161721) is a therapeutic, humanized monoclonal antibody (mAb) that targets the human IL-21 (interleukin 21) [1]. This antibody was engineered to feature the M252Y/S254T/T256E triple mutation to enhance its bindind the the neonatal fragment crystallizable receptor (FcRn) and increase its half-life in plasma [1]. By binding to IL-21, Avizakimab prevents it from interacting with its receptor on the surface of immune cells, thus inhibiting its downstream functions, notably T cell and B cell proliferation and differentiation. Avizakimab is expected to induce T-cell exhaustion and tolerance in vivo [1].
IL-21 is a member of the IL-2/γc cytokine superfamily. It has pleiotropic actions on a wide range of immune (i.e. lymphoid and myeloid populations) and non-immune cell types (i.e. epithelial cells). IL-21 is referred to as a double-edged sword cytokine, playing key roles in both anti-tumor and anti-viral responses, as well as in promoting the development of autoimmune diseases (e.g., systemic lupus erythematosus and rheumatoid arthritis) [2].
Avizakimab has been clinically evaluated as an immunosuppressive agent for the treatment of systemic lupus erythematosus (SLE) [3].
References:
1. Hussaini A., et al., 2019. A Double-Blind, Phase I, Single Ascending Dose Study to Assess the Safety, Pharmacokinetics, and Pharmacodynamics of BOS161721 in Healthy Subjects. Clinical and Translational Science, 13(2):337.
2. Long D. et al., 2019. Clinical significance and immunobiology of IL-21 in autoimmunity. J Autoimmunity. 99:1.
3. NCT03371251 https://clinicaltrials.gov (accessed October 2025).
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?