The BAFF (B cell activating factor) and APRIL (A Proliferation-Inducing Ligand) cytokines are central regulators of B cell survival and antibody production. Yet their dysregulation can drive autoimmunity and hematological cancers. Here we discuss how this cytokine signaling axis shapes immune-mediated inflammatory diseases (IMIDs). We also outline its role in supporting malignant cell proliferation and highlight its emerging functions outside classical B cell biology.
BAFF/APRIL signaling in B cells
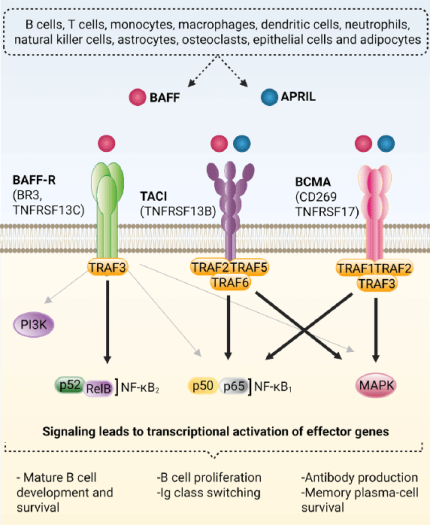
BAFF (TNFSF13B, BLyS) and APRIL (TNFSF13) originate from a broad array of cell types. Besides their well-known production by immune cells (e.g. B and T cells, macrophages), both cytokines are also released by non-immune cells (e.g. osteoclasts, epithelial cells, adipocytes)1,2. These cytokines exert their biological effects through three receptors: BAFF-R (B cell Activating Factor Receptor), TACI (Transmembrane Activator and CAML Interactor), and BCMA (B cell Maturation Antigen), whose expression, binding affinities, and downstream signaling shape B cell responses (Fig. 1).
- BAFF-R mainly activates the non-canonical NF-κB2 pathway. In resting cells, a TRAF3/2/cIAP1/2 complex continually degrades NIK. BAFF binding redirects this complex to degrade TRAF3 instead, stabilizing NIK and enabling IKK1-dependent processing and assembly of the p52/RelB transcription factor. This signaling cascade supports the survival and maturation of B cells. BAFF-R can also activate PI3 kinases, ERK1/2 MAP kinases, and the canonical NF-κB1 pathway, though these routes are less described3-5.
- TACI signals primarily through canonical NF-κB1 and JNK/MAP kinases pathways. Ligand binding induces TRAF2/5/6 recruitment, enabling IKK2-dependent processing and assembly of the p50/p65 and AP1 transcription factors. This cascade leads to B cell activation, proliferation, and immunoglobulin (Ig) class switching6-7.
- BCMA preferentially activates canonical NF-κB1 and MAP kinases pathways. Ligand binding induces TRAF1/2/3 recruitment, enabling p50/p65 assembly and ERK1/2, JNK, and p38 MAP kinases activation. These pathways reinforce plasma-cell survival mechanisms and sustained antibody (Ab) secretion8
From homeostasis to pathology
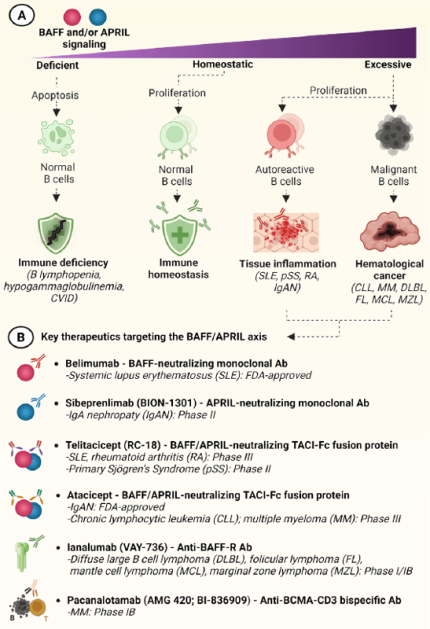
In homeostatic conditions, BAFF and APRIL maintain stable B cell proliferation and support Ab production. Impaired signaling, due to genetic defects or therapeutic blockade, is linked to immunodeficiencies like early B-cell loss (B-lymphopenia), insufficient Ab production (hypogammaglobulinemia), or a combined failure of both known as common variable immunodeficiency9,10 (Fig. 2A).
Conversely, excessive BAFF/APRIL signaling, often driven by chronic inflammation, promotes abnormal B-cell activation, prolonged survival, and uncontrolled Ab production. This persistent signaling sustains autoreactive B cells that, if not regulated, trigger autoimmune and inflammatory diseases1. In systemic lupus erythematosus (SLE), self-reactive B cells produce immune complexes, leading to localized tissue inflammation. In primary Sjögren’s syndrome (pSS), elevated BAFF and APRIL levels induce the formation of ectopic germinal centers within salivary glands, which are progressively destroyed by local autoreactive B cells. In rheumatoid arthritis (RA), BAFF-driven B-cell activation intensifies synovial inflammation, contributing to cartilage erosion and joint deformity9. IgA nephropathy (IgAN) represents an autoimmune-like renal disease where excessive APRIL signaling induces the production of aberrantly glycosylated IgA1 and immune complexes, triggering chronic glomerular inflammation10 (Fig. 2A).
In oncologic settings, malignant B cells engage BAFF/APRIL pathways for survival. In chronic lymphocytic leukemia (CLL), tumor B cells rely on BAFF-R, TACI, and BCMA signaling to resist apoptosis and persist within lymphoid tissues. In multiple myeloma (MM), APRIL/BCMA signaling activates NF-κB and MAP kinases pathways, promoting plasma-cell proliferation, survival, and drug resistance2. Similar mechanisms contribute to diffuse large B-cell lymphoma (DLBL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL) (Fig. 2A).
Together, these disorders illustrate how dysregulated BAFF/APRIL signaling shifts the B-cell compartment from immune protection to chronic inflammation or malignant expansion2.
Although these modalities differ in spectrum and selectivity, they share a mechanistic aim: restoring B-cell homeostasis by rebalancing ligand–receptor signals that support autoreactive and malignant B cells proliferation. Remaining challenges include defining BAFF- versus APRIL-dominant disease states, identifying predictive biomarkers, as well as refining approaches that inhibit pathologic signaling while maintaining protective immunity.
Beyond B cells: expanding the BAFF/APRIL horizon
Although traditionally linked to B-cell biology, BAFF and APRIL also act on non-immune cells. Indeed, BAFF-R, TACI, and BCMA are also expressed on adipocytes, osteoclasts, and gut epithelial cells, indicating broader roles in tissue communication. In adipose tissue, BAFF signaling promotes macrophage infiltration and inflammatory cytokine release, while both BAFF and APRIL influence diet-induced weight gain and energy balance through adipocyte signaling14. In gut epithelial and bone environments, excessive APRIL activity has been associated with inflammatory bowel disease and osteoporosis, by enhancing mucosal inflammation and osteoclast activation15,16.
Collectively, these findings also position the BAFF/APRIL axis at the interface of immunometabolism and tissue homeostasis. This expanded biology underscores the importance of dissecting BAFF and APRIL receptor-specific signaling in non-lymphoid tissues. Such insights could broaden therapeutic strategies beyond IMIDs and hematological cancers, such as metabolic and bone diseases.
Therapeutic targeting
Therapeutic modulation of BAFF/APRIL signaling has become central to managing B cell IMIDs and malignancies (Fig. 2B):
- BAFF-neutralizing Abs are suited to conditions in which elevated BAFF sustains autoreactive transitional and naïve B cells. By reducing BAFF-dependent survival signals, these agents contribute to apoptosis of B cells while sparing memory plasma cells1,11.
- APRIL-neutralizing Abs target disorders driven by dysregulated IgA biology. Because APRIL promotes IgA class switching and mucosal plasma-cell expansion, its inhibition can reduce aberrant IgA1 synthesis and immune-complex formation in IgAN12.
- TACI-Fc fusion proteins allow dual BAFF and APRIL blockade, thus offering broader suppression than specific Abs. These agents neutralize both ligands and reduce downstream survival signaling in naïve, transitional, and plasma-cell compartments, making them useful in diseases where both cytokines contribute to pathology1,2.
- BAFF-R–targeting Abs deplete BAFF-dependent B-cell subsets without affecting plasma cells, providing a focused strategy to reduce autoreactive or inflammatory proliferation while retaining humoral immunity2.
- Anti-BCMA-CD3 bispecific Abs induce efficient T cell-mediated lysis of malignant plasma cells.13
*Illustrations created with BioRender.com.
References
1. Balasubramaniam M. & Mokhtar A.M.A., 2024. Cell Signal. 120:110–120.
2. Ullah M.A. & Mackay F., 2023. Cancers. 15(6):1791.
3. Schweighoffer E. & Tybulewicz V.L., 2018. Curr Opin Cell Biol. 51:8–14.
4. Schweighoffer E. & Tybulewicz V.L., 2021. Curr Opin Immunol. 71:124–131.
5. Smulski C.R. & Eibel H., 2018. Front Immunol. 9:2285.
6. Kanno Y. et al., 2010. J Recept Signal Transduct. 30(2):121–132.
7. Xia X.Z. et al., 2000. J Exp Med. 192(1):137–144.
8. Hatzoglou A. et al., 2000. J Immunol. 165(3):1322–1330.
9. Sevdali E. et al., 2021. Curr Opin Immunol. 71:103–110.
10. Yeh T.W. et al., 2020. J Allergy Clin Immunol. 146(5):1109–1120.e4.
11. Dennis G.J., 2012. Clin Pharmacol Ther. 91(1):143–149.
12. Mathur M. et al., 2024. N Engl J Med. 390(1):20–31.
13. Rodriguez et al., 2025. Leuk & Lymph. 66(11):2108-2117.
14. Chan C.C. et al., 2021. Nat Commun. 12(1):1–11.
15. Kumric M. et al., 2021. Diagnostics. 11(1):1–15.
16. Hemingway F. et al., 2011. Bone. 48(5):938–944.

Tools for research
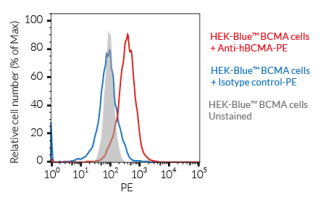
InvivoGen’s HEK-Blue™ BAFF-R, TACI, and BCMA reporter cell lines provide reliable systems to quantify receptor activation through an NF-κB/AP-1–inducible SEAP readout. Each line expresses a specific human receptor, enabling precise evaluation of cellular responses. Complementing these tools, our mammalian-produced recombinant human bioactive BAFF and APRIL preserve native glycosylation and bona fide 3D structure, with each lot validated using HEK-Blue™ cells. We also supply BAFF-, APRIL-, and BCMA/CD3-targeting antibodies to support mechanistic studies.
See featured products bellow: