Recombinant human GM-CSF (E. coli)
-
Cat.code:
rcyec-hgmcsf
- Documents
ABOUT
Human GM-CSF protein - E. coli-expressed, tag-free, carrier-free
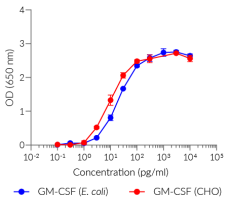
Recombinant human GM-CSF is a high-quality and biologically active cytokine, validated using proprietary GM-CSF reporter cells. This common β chain family member is produced in E. coli and thoroughly purified to remove endotoxins.
Recombinant human GM-CSF can be used together with HEK-Blue™ GM-CSF cells for the screening of inhibitory molecules, such as Mavrilimumab, a therapeutic monoclonal antibody targeting the α subunit of the GM-CSF receptor (GM-CSFR) (see figures).
Key features
- Each lot is validated using HEK-Blue™ GM-CSF cells
- Endotoxin < 0.01 EU/µg
- 0.22 µm sterile-filtered
Applications
- Standard for GM-CSF detection and quantification assays
- Screening and release assays for antibodies blocking GM-CSF signaling
- Screening and release assays for engineered GM-CSF
GM-CSF, also known as colony-stimulating factor 2 (CSF2), belongs to the β-common chain cytokine family. Although originally identified as a hematopoietic growth factor, this cytokine is now regarded as a pleiotropic regulator of inflammation in response to pathogens, autoimmune diseases, and cancer.
All products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
P04141
100 μg/ml in water
Phosphate buffer saline (pH 7.4)
The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK‑Blue™ TLR4 cells.
Cellular assays
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Recombinant human GM-CSF (E. coli)
-
Cat code:rcyec-hgmcsf
-
Quantity:20 µg
1.5 ml endotoxin-free water
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
GM-CSF background
The granulocyte–macrophage colony-stimulating factor (GM-CSF), also known as colony-stimulating factor 2 (CSF2), belongs to the β-common chain cytokine family [1]. It promotes the differentiation, activation, and survival of cells from the myeloid compartment, notably macrophages, dendritic cells, and neutrophils [2, 3]. Although originally identified as a hematopoietic growth factor, this cytokine is now regarded as a pleiotropic regulator of inflammation in response to pathogens, autoimmune diseases, and cancer [2,4].
GM-CSF signalization requires a multimeric structure comprising four α chains (GMRα, aka CD116), four β chains (GMRβ, CD131), and four cytokines. This 12-protein complex allows the juxtaposition of the intracellular Janus kinase 2 (JAK2) and activation of the signal transducer and activator of transcription 5 (STAT5) [1]. Other signaling pathways include ERK, NF-κB, and AKT pathways [1, 5]. The understanding of cellular and molecular mechanisms whereby GM-CSF exerts its varied functions is key for the development of therapeutic strategies (e.g. cancer vaccines, blocking antibodies) [1].
Mavrilimumab is a fully human IgG4 monoclonal antibody that targets the alpha subunit of the granulocyte–macrophage colony-stimulating factor receptor (GM-CSFR), effectively blocking GM-CSF signaling [2]. As of June 2025, Mavrilimumab (CAM-3001) is not yet FDA-approved. It is still under clinical investigation for the treatment of various autoimmune diseases, including rheumatoid arthritis (RA) and giant cell arteritis (GCA) [3, 6].
References:
1. Dougan M. et al., 2019. GM-CSF, IL-3, and IL-5 family of cytokines: regulators of inflammation. Immunity. 50(4):796-811.
2. Burmester GR, et al., 2011. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann Rheum Dis. ;70(9):1542-9.
3. Burmester GR, et al. 2017. A randomised phase IIb study of mavrilimumab, a novel GM-CSF receptor alpha monoclonal antibody, in the treatment of rheumatoid arthritis. Ann Rheum Dis. 76(6):1020-1030.
4. Zhan Y. et al., 2019. The Pleiotropic Effects of the GM-CSF Rheostat on Myeloid Cell Differentiation and Function: More Than a Numbers Game. Front Immunol. 102679.
5. Hamilton J.A., 2020. GM-CSF in inflammation. J. Exp . Med. 217(1):e20190945.
6. Cid MC, et al., 2022. Efficacy and safety of mavrilimumab in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022 May;81(5):653-661.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?