Ruxolitinib
-
Cat.code:
tlrl-rux-3
- Documents
ABOUT
JAK1 and JAK2 inhibitor
Ruxolitinib (also known as INCB018424) is a potent, reversible, and selective Janus Kinase (JAK) 1 and JAK2 inhibitor [1, 2]. Ruxolitinib was developed as a potential therapeutic for a family of blood cancers termed myeloproliferative neoplasms (MPNs), which are characterized by the aberrant activation of the JAK-STAT pathway due to a mutation (V617F) in JAK2 [1,3].
![]() More details on Janus Kinase (JAK)
More details on Janus Kinase (JAK)
Mode of action:
Ruxolitinib competes with ATP for binding to the catalytic site in the kinase domain and thus inhibits not only the mutated JAK2 but wild-type JAK1-2 signaling pathways. Inhibition of the JAK-STAT signaling pathway by Ruxolitinib results in a dramatic decrease in levels of inflammatory cytokines, such as IL-6 and TNF-α. It is with this attenuation of the inflammatory response that the clinical efficiency of Ruxolitinib is attributed [2].
Ruxolitinib is approved for the treatment of the MPNs, myelofibrosis, and polycythemia vera [3]. Pre-clinical data suggest that Ruxolitinib shows potential in the treatment of inflammatory conditions such as acute graft versus host disease (GvHD) [4]. Additionally, synergy has been reported between Ruxolitinib and the chemotherapy drug, dexamethasone, in the treatment of acute lymphoblastic leukemia (ALL) in both in vitro and in vivo pre-clinical models [5].
Key features:
- Specific and potent JAK1 and JAK2 inhibitor
- InvitroFit™ grade: highly pure (≥97%) and inhibitory function validated in cellular assays
References
1. Quintas-Cardama A. et al., 2010. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115, 3109-3117.
2. Ajayi S. et al., 2018. Ruxolitinib. Recent Results Cancer Res 212, 119-132.
3. Mascarenhas J. & Hoffman R., 2012. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res 18, 3008-3014.
4. Zeiser R. et al., 2020. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med 382, 1800-1810.
5. Verbeke D. et al., 2019. Ruxolitinib Synergizes With Dexamethasone for the Treatment of T-cell Acute Lymphoblastic Leukemia. Hemasphere 3, e310.
All products are for research use only, and not for human or veterinary use.
InvitroFit™
InvitroFit™ is a high-quality standard specifically adapted for in vitro studies of inhibitors. InvitroFit™ products are highly pure (≥95%) and guaranteed free of bacterial contamination, as confirmed using HEK Blue™ TLR2 and HEK Blue™ TLR4 cellular assays. Each lot is rigorously tested for functional activity using validated (or proprietary) cellular models. This grade ensures reliability and reproducibility for your research applications.
SPECIFICATIONS
Specifications
C17H18N6
10 mg/ml in DMSO
100 nM-10 μM for cell culture assays
Negative (tested using EndotoxDetect™ assay)
In vitro cellular assays
Each lot is functionally tested and validated using cellular assays.
CONTENTS
Contents
-
Product:Ruxolitinib
-
Cat code:tlrl-rux-3
-
Quantity:3 x 5 mg
Ruxolitinib is provided as a translucent film.
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Janus kinases
Janus kinases (JAKs) are constitutively bound to cytokine receptors, such as Type I interferons (IFNs) and interleukin-6. Upon binding of the ligand to the receptor, JAKs phosphorylate downstream targets such as STAT3/5, Akt, and ERK. Ultimately, this induces the production of cytokines and chemokines, including IFN-stimulated genes (ISGs). JAK-STAT signaling is crucial for the regulation and homeostasis of hematopoiesis and immunity [2].
1. Quintas-Cardama A. et al., 2010. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115, 3109-3117.
2. Ajayi S. et al., 2018. Ruxolitinib. Recent Results Cancer Res 212, 119-132.
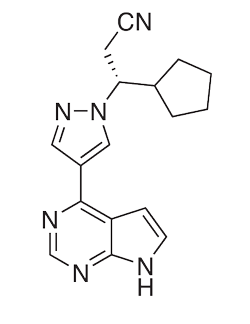
Chemical structure of Ruxolitinib
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Validation Data Sheet
Certificate of analysis
Need a CoA ?