c-di-GMP VacciGrade™
-
Cat.code:
vac-nacdg
- Documents
ABOUT
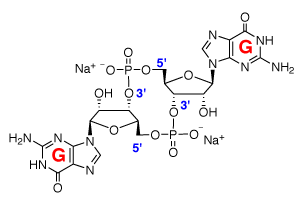
Cyclic diguanylate monophosphate - STING agonist - CAS #2222132-40-1
Cyclic diguanylate monophosphate (c-di-GMP) is a cyclic dinucleotide (CDN) produced by bacteria in which it functions as an essential second messenger. It controls motility, biofilm formation, and bacterial pathogenicity.
CDNs have been shown to increase vaccine potency [1]. They activate innate immunity by directly binding the endoplasmic reticulum-resident receptor STING (stimulator of interferon genes), activating a signaling pathway that induces the expression of interferon-β (IFN-β) and also nuclear factor-κB (NF-κB) dependent inflammatory cytokines [2, 3].
c-di-GMP exerts strong adjuvant activities when delivered by the mucosal route [4, 5]. It elicits a balanced Th1/Th2 and Th17 response, which is crucial against intracellular pathogens [6].
c-di-GMP VacciGrade™ is a high-quality pre-clinical grade.
![]() Read our review on STING: Deciphering the STING Paradox
Read our review on STING: Deciphering the STING Paradox
References:
1. Dubensky TW. et al., 2013. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Therapeutic Advances in Vaccines 1(4): 131.
2. Jin L. et al., 2011. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J Immunol. 187(5):2595.
3. Burdette DL. et al., 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 478(7370):515.
4. Neuhaus V. et al., 2014. A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung. Vaccine. 32(26):3216.
5. Blaauboer SM. et al., 2014. MPYS/STING-mediated TNF-α, not type I IFN, is essential for the mucosal adjuvant activity of (3'-5')-cyclic-di-guanosine-monophosphate in vivo. J Immunol. 192(1):492.
6. Madhun AS. et al., 2011. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine.29(31):4973.
All products are for internal research use only, and not for human or veterinary use.
VacciGrade™
VacciGrade™ is a high-quality pre-clinical grade, suitable for in vivo studies. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay.
SPECIFICATIONS
Specifications
STING
C20H22N10O14P2•2Na
50 mg/ml in water
Sterility guaranteed
< 0.005 EU/μg
Cellular assays for type I interferon induction
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:c-di-GMP VacciGrade™
-
Cat code:vac-nacdg
-
Quantity:1 mg
10 ml sterile endotoxin-free physiological water (NaCl 0.9%)
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Validation Data Sheet
Certificate of analysis
Need a CoA ?