2’3’-cGAMP VacciGrade™
-
Cat.code:
vac-nacga23
- Documents
ABOUT
STING-based vaccine adjuvant - Th1/Th2 response
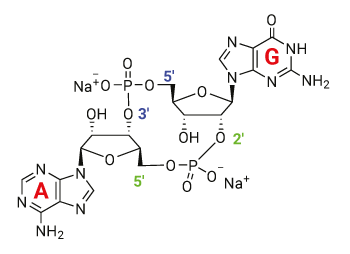
2’3’-cGAMP VacciGrade™ is a preclinical grade preparation of the cyclic dinucleotide 2’3’-cGAMP (cyclic [G(2’,5’)pA(3’,5’)p]), a cyclic dinucleotide (CDN) produced in mammalian cells by cGAS (cGAMP synthase) in response to double-stranded DNA in the cytoplasm.
2’3’-cGAMP is also referred to as “noncanonical” cGAMP due to the presence of the atypical 2’-5’ phosphodiester linkage between the guanosine and the adenosine. Structural and functional studies revealed that noncanonical 2’3’-cGAMP is distinct from the canonical 3’3’-cGAMP produced by bacteria [1, 2].
CDNs have been shown to increase vaccine potency [3]. CDNs activate innate immunity by directly binding the endoplasmic reticulum-resident receptor STING (stimulator of interferon genes), leading to the expression of interferon-β (IFN-β) and nuclear factor-κB (NF-κB) dependent inflammatory cytokines.
2′3′-cGAMP is an effective adjuvant that boosts the production of antigen-specific antibodies and T cell responses in mice [4].
2’3’-cGAMP VacciGrade™ is a high-quality pre-clinical grade.
![]() Read our review on STING: Deciphering the STING Paradox
Read our review on STING: Deciphering the STING Paradox
References:
1. Diner E. et al., 2013. The Innate Immune DNA Sensor cGAS Produces a Noncanonical Cyclic Dinucleotide that Activates Human STING. Cell Rep. 3(5):1355-61.
2. Gao P. et al., 2013. Cyclic [G(2',5')pA(3',5')p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 153(5):1094-107.
3. Dubensky TW. et al., 2013. Rationale, progress, and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Therapeutic Advances in Vaccines 1(4): 131-143.
4. Li XD. et al., 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 341(6152):1390-4.
All products are for research use only, and not for human or veterinary use.
VacciGrade™
VacciGrade™ is a high-quality pre-clinical grade, suitable for in vivo studies. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay.
SPECIFICATIONS
Specifications
STING
C20H22N10O13P2 •2Na
cyclic [G(2’,5’)pA(3’,5’)p]
50 mg/ml in physiological water
Sterility guaranteed
< 5 EU/mg (measurement by kinetic chromogenic LAL assay)
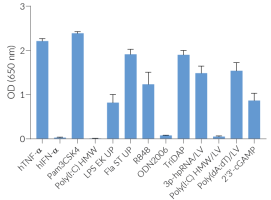
Induction of the interferon pathway in cellular assays
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:2’3’-cGAMP VacciGrade™
-
Cat code:vac-nacga23
-
Quantity:1 mg
10 ml sterile endotoxin-free physiological water (NaCl 0.9%)
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Validation Data Sheet
Certificate of analysis
Need a CoA ?