HEK-Blue™ IL-18 Cells
-
Cat.code:
hkb-hmil18
- Documents
ABOUT
IL-18 responsive NF-κB/AP1-SEAP reporter assay
HEK-Blue™ IL-18 cells are designed to monitor interleukin 18 (IL-18)-induced NF-κB and AP-1 stimulation or inhibition. This colorimetric bioassay can be used for screening activatory molecules, such as engineered cytokines, or inhibitory molecules, such as neutralizing antibodies.
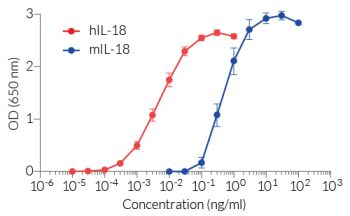
HEK-Blue™ IL-18 cells respond specifically to recombinant human IL-18. They also detect recombinant mouse IL-18 with lower sensitivity. The reliable and consistent performance of HEK-Blue™ IL-18 cells makes them suitable for release assays of therapeutic molecules that inhibit IL-18 signaling, such as Camoteskimab, a monoclonal antibody targeting IL-18 (see figures).
Key features
- Readily assessable NF-κB/AP1-SEAP reporter activity
- Convenient readout using QUANTI-Blue™ Solution
- High sensitivity to human (h) IL-18 and lower sensitivity to mouse (m) IL-18 activity
- Stability guaranteed for 20 passages
Applications
- Therapeutic development
- Drug screening
- Release assay
Interleukin-18 (IL-18) is a pro-inflammatory cytokine and a powerful inducer of type 1 responses mediated by activated NK, Th1, and CD8+ cytotoxic T cells. Excessive IL-18 signaling, either due to gain-of-function mutations or inefficient negative regulation, is linked to inflammatory disorders (e.g. macrophage activation syndrome (MAS), systemic juvenile idiopathic arthritis (sJIA), adult-onset Still’s disease (AOSD), metabolic syndrome) and autoimmune diseases (e.g. Crohn’s disease, systemic lupus erythematosus (SLE)).
Disclaimer: These cells are for internal research use only and are covered by a Limited Use License (See Terms and Conditions). Additional rights may be available.
SPECIFICATIONS
Specifications
IL-18
Human, Mouse
Detection and quantification of IL-18 activity
1 pg - 1 ng/ml (hIL-18)
200 pg - 30 ng/ml (mIL-18)
Complete DMEM (see TDS)
Verified using Plasmotest™
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:HEK-Blue™ IL-18 Cells
-
Cat code:hkb-hmil18
-
Quantity:3-7 x 10^6 cells
- 2 x 1 ml of HEK-Blue™ Selection (250x concentrate)
- 1 ml of Normocin® (50 mg/ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
Shipping & Storage
- Shipping method: Dry ice
- Liquid nitrogen vapor
- Upon receipt, store immediately in liquid nitrogen vapor. Do not store cell vials at -80°C.
Storage:
Caution:
Details
Cell line description
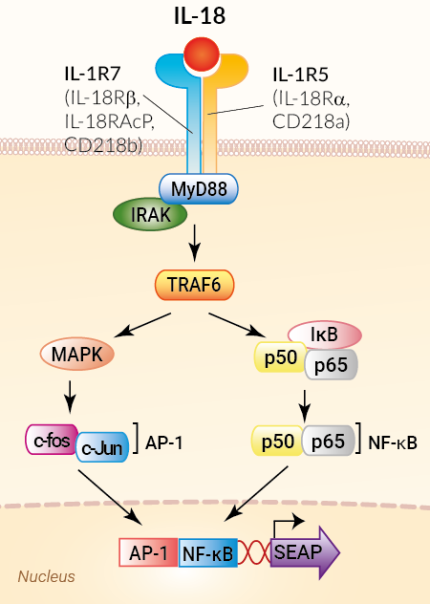
HEK-Blue™ IL-18 cells were generated by stable transfection of the human embryonic kidney HEK293 cell line with the genes encoding the IL-18 receptor (IL-18R) and IL-18 receptor accessory protein (IL-18RAP). These cells were further transfected with an NF-κB/AP-1-inducible secreted embryonic alkaline phosphatase (SEAP) reporter. The binding of IL-18 to its heterodimeric IL-18 receptor triggers a signaling cascade leading to NF-κB/AP-1 activation and the subsequent production of SEAP. This can be readily assessed in the supernatant using QUANTI-Blue™ Solution, a SEAP detection reagent.
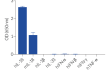
HEK-Blue™ IL-18 cells are highly sensitive to human (h) IL-18 and also respond to a lesser extent to murine (m) IL-18. These cells are non‑responsive to hIL-1β, hIL-33, hIFN-α, hIFN-β , hIFN-γ, and hTNF-α (see figures).
IL-18 background
Interleukin-18 (IL-18) is a member of the IL-1/Toll-like receptor cytokine superfamily, a group of cytokines that play important roles in host defense, immune regulation, and inflammation [1-3]. It is principally produced by macrophages, dendritic cells, endothelial cells, and epithelial cells. Like IL-1β, it is synthesized as an inactive precursor that is cleaved and processed into its mature bioactive form by caspase-1 upon inflammasome activation [1, 2].
IL-18 mediates its biological effects through the IL-1R5 receptor (also known as IL-18Rα, CD218a) and the IL-1R7 accessory protein (also known as IL-18RAcP, IL-18β, CD218b) [1, 2, 4]. Upon ligand binding, IL-1Rs dimerize through their Toll/interleukin-1 resistance (TIR) domains to recruit the MyD88 adaptor protein, which then couples to IL-1R-associated kinases (IRAKs) and tumor necrosis factor receptor-associated factor 6 (TRAF6). This leads to the activation of key transcription factors, including NF-κB, AP-1, and IRFs [1-4].
Formerly known as interferon-γ (IFN-γ) inducing factor, IL-18 drives pro-inflammatory responses from both the innate and adaptive immune systems [1-3]. It is primarily described as a potent inducer of NK (natural killer) cell activation and the polarization of CD4+ T-helper cells into Th1 or Th2 types, depending on the surrounding cytokine milieu. In addition, IL-18 exhibits characteristics of other proinflammatory cytokines, including increases in cell adhesion molecules, nitric oxide synthesis, and chemokine production. IL-18 plays a prominent role in fighting microbial infections caused by viruses, intracellular bacteria, or fungi.
The activity of mature IL-18 is controlled by the IL-18BP (IL-18 binding protein), which functions as a decoy receptor, preventing IL-18 from engaging with the signaling receptor [1-3].
Relevance for therapeutics development
Excessive IL-18 signaling, either due to gain-of-function mutations or inefficient negative regulation, is linked to inflammatory disorders (e.g., macrophage activation syndrome (MAS), systemic juvenile idiopathic arthritis (sJIA), adult-onset Still’s disease (AOSD), metabolic syndrome) and autoimmune diseases (e.g., Crohn’s disease, systemic lupus erythematosus (SLE)) [1, 2].
Therapeutic strategies include recombinant human IL-18BP (Tadekinig alpha) and an anti-human IL-18 monoclonal antibody (Camoteskimab) to neutralize IL-18 signaling. Conversely, DR-18 (ST-067), an IL-18 mutein resistant to IL-18BP decoy receptor, has shown promising results in augmenting immune responses to cancer [1].
References:
1. Landy, E., et al., 2024. Biological and clinical roles of IL-18 in inflammatory diseases. Nat Rev Immunol. 20(1):33-47.
2. Kaplancki, G., et al., 2017. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev. 281(1):138-153.
3. Mantovani, A., et al., 2019. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity. 50(4): p. 778-795.
4. Gaballa, J.M., et al., 2024. International nomenclature guidelines for the IL-1 family of cytokines and receptors. Nature Immunology. 25(4): p. 581-582.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?
You may also need
FAQ
HEK293 cell line description
Only our HEK-Blue™ Null1-k and Null2-k cells are sensitive to G418.
The only HEK-Blue™ Null cells that are sensitive to Puromycin are the HEK-Blue™ Null1-v cells.
The only HEK-Blue™ Null cells that are sensitive to Puromycin are the HEK-Blue™ Null1-v cells.
Our RNAseq data confirms that our HEK-Blue™ cells express FcRN, however, this has not been functionally tested.
The difference in activity is approximately 10-fold.
Both cell types overexpress a designated TLR. The primary difference is that HEK-Blue™ cells include the NFκB inducible SEAP reporter construct.
We presume them to be in the endosome as we express the wild-type, full-length genes. Additionally, their signal can be blocked by Chloroquine, an endosomal acidification inhibitor. However, as these TLRs are over-expressed there may potentially be low expression of them on the cell surface.
HEK-Blue™ cells only express a single NFκB inducible SEAP reporter gene. Whereas, HEK-Dual™ cells have the addition of the Lucia™ gene knocked into the IL-8 locus. Thus, when IL-8 is activated following stimulation, HEK-Dual™ cells can report this with the secretion of Lucia™ luciferase.
It should also be noted that the HEK-Dual™ cells have been knocked out for TLR3, TLR5, and TNFR to limit interference from other TLRs when studying a specific TLR pathway.
HEK293 cells express TLR1, TLR3, TLR5, TLR6, and NOD1.
They respond to TLR3, TLR5, and NOD1 agonists, but at a much lower level compared to HEK293 cells transfected with these receptors
HEK-Blue™ IL-1R cells express both human and murine IL-1β receptors, thus can detect both species.
On the other hand, HEK-Blue™ IL-1β cells are specific for human IL-1β, but can still detect higher concentrations of mouse IL-1β.
In the United States, HEK293 cell lines are designated Biosafety Level 2 according to the Center for Disease Control and Prevention (CDC).
In Germany, HEK293 cell lines are designated Biosafety Level 1 according to the Central Committee of Biological Safety, Zentrale Kommission für die Biologische Sicherheit (ZKBS).
You can check with your country’s regulatory authority regarding the use of these cells.
Please note that there is no replicating/infectious Adenovirus 5 in these cells.
The minimal promoter isn't the same but the difference in expression for these two Null cell lines is minor.
There is no specific integration system used to generate our stable cell lines.
The selection pressure is enough to obtain stable clones. The receptors are added by simple transfection of plasmids using a cationic lipidic transfection agent (the plasmids are not linearized before transfection).
Cell line culture
HEK-Blue™ cells should be seeded at a density of approximately 1.5 x 106 cells in a T25 flask or 4 – 5 x 106 cells in a T75 flask.
We recommend using a flat-bottom, clear walled cell culture plate.
Below are a few tips we recommend to help get your HEK cells growing:
• For the first 2-3 passages, grow cells in media containing 20% FBS and no antibiotics.
• Do not allow cells to reach 100% confluency Please check cells as regularly as possible.
• The cells should not be grown in 20% FBS for too long. Use media with 10% FBS after 2 or 3 passages.
• When making frozen stocks, continue growing additional cultures in case there is a problem with the frozen stock.
Trypsin does not adversely affect the health or growth of these cells. However, it is known that high concentrations will occasionally induce the activation of NFκB resulting in a higher background in your assay.
Moreover, we have observed some cases where trypsin has been contaminated with TLR2, TLR4, and TLR5 contaminants, which can also interfere with the assay results.
It is not unusual for different TLR cells to grow at different rates. Some TLR clones happen to grow a little slower/faster than others. This is often clone dependent.
When the HEK-Blue™ cells are non-adherent, either they were diluted too harshly at the start or they have grown over-confluent in a small flask and suffocated.
To avoid this in the future:
• Change the media and plate the cells at a density of approximately 1.5 x 106 cells in a T25 flask.
• Wash the cells before putting them into a new flask. Sometimes when the cells are non-adherent, it is due to the clustering of both live and dead cells. Additionally, this will get rid of any remaining DMSO which could affect the adhesion of the cells to the flask.
• Use medium with 20% FBS.
• The use of CellBIND flasks can sometimes help to increase attachment and growth of the cells (however CellBIND flasks are not required in the normal protocol).
The split ratio will depend on when you expect confluency. Typically, the doubling time of HEK-Blue™ cells is approximately 24 hours.
Therefore, if you use a split ratio of 1:2 (50%) into a new flask, cells should be confluent the following day. If you use a split ratio of 1:4 (25%) you can expect the cells to be confluent after 2 days.
Our HEK-Blue™ Selection is provided in 1ml tubes with each containing a 250X solution.
Therefore, you should dilute HEK-Blue™ Selection 1:250 into your media to have a 1X concentration.
Assays
HEK293 cells are very easy to transfect with a transfection efficiency of approximately 80%.
It depends on the cell line and the concentration of the ligand used to stimulate the cells. In general, we record the results following 16 – 24 hours of stimulation.
There are 2 possible explanations as to why a blue color is observed in all wells.
1. It could be due to the presence of Alkaline Phosphatase (AP) in the culture medium. To see if this is the case, there is a very simple test to perform. Add 50 µl of the medium used for cell culture (without cells) and 200 µl of resuspended HEK-Blue™ detection medium or QUANTI-Blue™. If the medium turns blue, then it is due to the presence of Alkaline Phosphatase (AP) in the serum of the media. In this case, you must heat the serum to inactivate the AP and repeat the medium test. At this point the test should give a negative result (no blue color).
2. It could be due to improper handling of cells before the test. To avoid activation of NFκB before stimulation and reduce the risk of false positive results:
• Use pre-warmed PBS to wash cells
• Use heat-inactivated FBS
• Do not centrifuge cells prior to stimulation
• Do not use trypsin
We have noticed a loss of sensitivity when using HEK-Blue™ Detection medium instead of QUANTI-Blue™ on our cytokine reporter cell lines.
Therefore, we recommend using QUANTI-Blue™, which is provided with the cells, as this is what we use in house.
We recommend to not use any antibiotics at all during assays to ensure the least amount of potential interfering agents in the medium.
Therefore, we do not add HEK-Blue™ Selection to the test media.
We have only tested the use of plasma and serum samples on our HEK-Blue™ hTLR2 cell line.
The results demonstrated that when compared to using standard samples (in DMEM), serum samples give a single log difference.
On the other hand, we found a 3-log difference between DMEM and plasma samples.
This is why we would recommend using serum samples over plasma samples.
Yes, they can be used interchangeably. However, please note that the protocols are distinctly different and need to be followed accordingly.