Anti-hIFN-α-hIgG1
-
Cat.code:
hifna-mab1
- Documents
ABOUT
Anti-human IFN alpha - Rontalizumab biosimilar - CAS #948570-30-7
Anti-hIFN-α-hIgG1 is a biosimilar antibody of Rontalizumab, a therapeutic antibody that targets interferon alpha (IFN-α). This monoclonal antibody (mAb) blocks the interaction of IFN-α with its receptor. Rontalizumab has been clinically evaluated as an immunosuppressive agent for the treatment of systemic lupus erythematosus (SLE).
Anti-hIFN-α-hIgG1 comprises the variable region of Rontalizumab and the human IgG1 constant region of Rontalizumab with high effector functions.
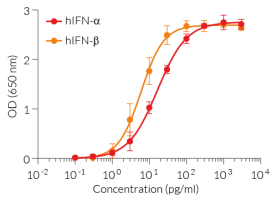
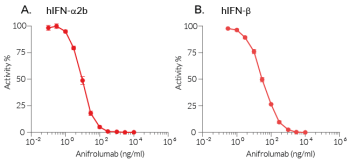
This antibody can be used with HEK-Blue™ IFN-α/β cells for screening and neutralization assays to block recombinant human IFN-α-induced signaling(see figure).
Key features
- Potent and specific neutralization activity against hIFN-α2
- Reacts with hIFN-α1, hIFN-α2, hIFN-α5, hIFN-α8, hIFN-α14, hIFN-α16, hIFN-α17, and hIFN-α21
- Weakly reacts with hIFN-α4 and IFN-α10
- Does not react with hIFN-α6 or hIFN-α7
- Each lot is functionally tested and validated.
- The complete sequence of the antibody construct was verified.
- The absence of endotoxins is determined using the EndotoxDetect™ assay.
All InvivoGen products are for internal research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
IFN-α
Human
Reacts with human IFN-α1, IFN-α2, IFN-α5, IFN-α8, IFN-α14, IFN-α16, IFN-α17 and IFN-α21. Very weakly reacts with human IFN-α4 and IFN-α10. It does not react with IFN-α6 or IFN-α7.
Blocking & Neutralization
Sodium phosphate buffer with glycine, saccharose, and stabilizing agents
0.2 µm filtration
Negative (tested using EndotoxDetect™ assay)
Neutralization assay, ELISA, Fc effector function analysis
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Anti-hIFN-α-hIgG1
-
Cat code:hifna-mab1
-
Quantity:100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Rontalizumab background
Rontalizumab (aka rhuMAb interferon-alpha) is a therapeutic, humanized monoclonal antibody (mAb) that targets the human interferon-alpha (IFN-α) cytokine subtypes [1, 2]. By binding to IFN-α, Rontalizumab prevents it from interacting with its receptor (IFNAR) on the surface of immune cells, thus inhibiting the downstream inflammatory response. Rontalizumab has been clinically evaluated as an immunosuppressive agent for the treatment of systemic lupus erythematosus (SLE) [1-3].
References:
1. McBride JM, et al. 2012. Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study. Arthritis Rheum. 64(11):3666-76.
2. Kalunian KC, et al., 2016. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-alpha) in patients with systemic lupus erythematosus (ROSE). Ann Rheum Dis. (2016) 75:196–202.
3. Jones, S.A. and Morand, E.F., 2024. Targeting Interferon Signalling in Systemic Lupus Erythematosus: Lessons Learned. Drugs. 84(6):625-635.
DOCUMENTS
Documents
Technical Data Sheet
Validation Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?