N-Glycolyl-MDP
-
Cat.code:
tlrl-gmdp
- Documents
ABOUT
NOD2 Agonist - N-glycolylated muramyldipeptide
N-Glycolyl-MDP is a synthetic N-glycolylated form of muramyl dipeptide (MDP). MDP is the minimal bioactive peptidoglycan (PGN) motif present in almost all bacteria. MDP was first identified as an active component in Freund’s complete adjuvant [3]. It is recognized by the cytosolic receptor NOD2 [1, 2].
Mode of action:
Most bacteria produce N-acetylated MDP in contrast to Mycobacteria which produce N-glycolylated MDP. In Mycobacterial PGN the N-acetyl group at the carbon 2 of the muramic acid is preferentially hydroxylated to an N-glycolyl group through action of the enzyme N-acetyl muramic acid hydroxylase (NamH). The cell wall of mycobacteria is known to be extremely immunogenic. This potent activity is attributed to their N-glycolylated MDP. NOD2 activation with N-Glycolyl-MDP causes pro-inflammatory cytokine release through the mitogen-activated protein kinase (MAPK) and NF-κB activation, thus contributing to host defense [1, 2].
Key features:
- Potent NOD2 agonist
- Synthetic N-glycolylated MDP
- Each lot is functionally tested
![]() Read our review on NOD-Like Receptors
Read our review on NOD-Like Receptors
References:
1. Coulombe F. et al., 2009. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 206(8):1709-16.
2. Hansen J.M. et al., 2014. N-glycolylated peptidoglycan contributes to the immunogenicity but not pathogenicity of Mycobacterium tuberculosis. C Infect Dis. 209(7):1045‑54.
3. Ogawa C. et al., 2011. Muramyl dipeptide and its derivatives: peptide adjuvant in immunological disorders and cancer therapy. Curr Bioact Compd. 7(3):180-97.
4. Raymond J.B. et al., 2005. Identification of the namH gene, encoding the hydroxylase responsible for the N-glycolylation of the mycobacterial peptidoglycan. J Biol Chem. 280(1):326-33.
All products are for research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
C19H32N4O12
100 ng/ml - 10 µg/ml
5 mg/ml in water
Activation of NOD2 using HEK-Blue™ NOD2 cells, Absence of NOD1 activity confirmed using HEK‑Blue™ NOD1 cells, Absence of bacterial contamination confirmed using HEK-Blue™ TLR2 and HEK‑Blue™ TLR4 cells
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:N-Glycolyl-MDP
-
Cat code:tlrl-gmdp
-
Quantity:5 mg
1.5 ml sterile endotoxin-free water
Shipping & Storage
- Shipping method: Room temperature
- Upon receipt, store at -20°C.
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
NOD1 and NOD2
The cytosolic NOD-Like Receptors (NLRs, also known as NODs or NALP) are Nucleotide-binding Oligomerization Domain containing receptors. To date, 22 NLRs have been identified in humans and constitute a major class of intracellular pattern recognition receptors (PRRs) [1].
The founding NLR-family members NOD1 (CARD4) and NOD2 (CARD15) recognize distinct motifs of peptidoglycan (PGN), an essential constituent of the bacterial cell wall. NOD1 senses the D-γ-glutamyl-meso-DAP dipeptide (iE-DAP), which is found in PGN of all Gram-negative and certain Gram-positive bacteria [1, 2] whereas NOD2 recognizes the muramyl dipeptide (MDP) structure found in almost all bacteria. Thus NOD2 acts as a general sensor of PGN and NOD1 is involved in the recognition of a specific subset of bacteria. Both iE-DAP and MDP must be delivered intracellularly either by bacteria that invade the cell or through other cellular uptake mechanisms. Ligand-bound NOD1 and NOD2 oligomerize and signal via the serine/threonine RIP2 kinase through CARD-CARD homophilic interactions [3]. Once activated, RIP2 mediates ubiquitination of NEMO/IKKγ leading to the activation of NF-κB and the production of inflammatory cytokines. Furthermore, poly-ubiquitinated RIP2 recruits TAK1, which leads to IKK complex activation and the activation of MAPKs [4].
Genetic mutations in NOD2 are associated with Crohn’s disease, a chronic inflammatory bowel disease [5]. In addition, numerous studies have recently revealed that NOD1 and NOD2 have a close relationship with a variety of cancers via controlling proliferation, altering immunosurveillance, and interacting with tissue bacteria, including intestinal commensal intestinal microflora. Moreover, additional research into the mechanisms of NOD1 and NOD2 in cancers would shed light on the innate immunity-cancer relationship and provide intriguing targets for immunotherapy [6].
References:
1. Chamaillard M. et al., 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4: 702-707.
2. Girardin S. et al., 2003. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300: 1584-1587.
3. Kobayash, K. et al., 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416: 194-199.
4. Kobayashi K. et al., 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307: 731-734.
5. Ogura Y. et al., 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411: 603-606.
6. Wang D., 2022. NOD1 and NOD2 Are Potential Therapeutic Targets for Cancer Immunotherapy. Comput Intell Neurosci.;2022:2271788.
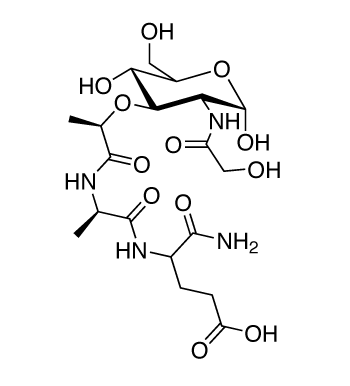
Structure of N-Glycolyl-MDP:
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Validation Data Sheet
Certificate of analysis
Need a CoA ?

