Spotlight on COVID-19: Vaccine development
Spotlight on COVID-19
• The infection cycle of SARS-CoV-2
• Treatment with repurposed drugs (updated)
COVID-19-RELATED PRODUCTS
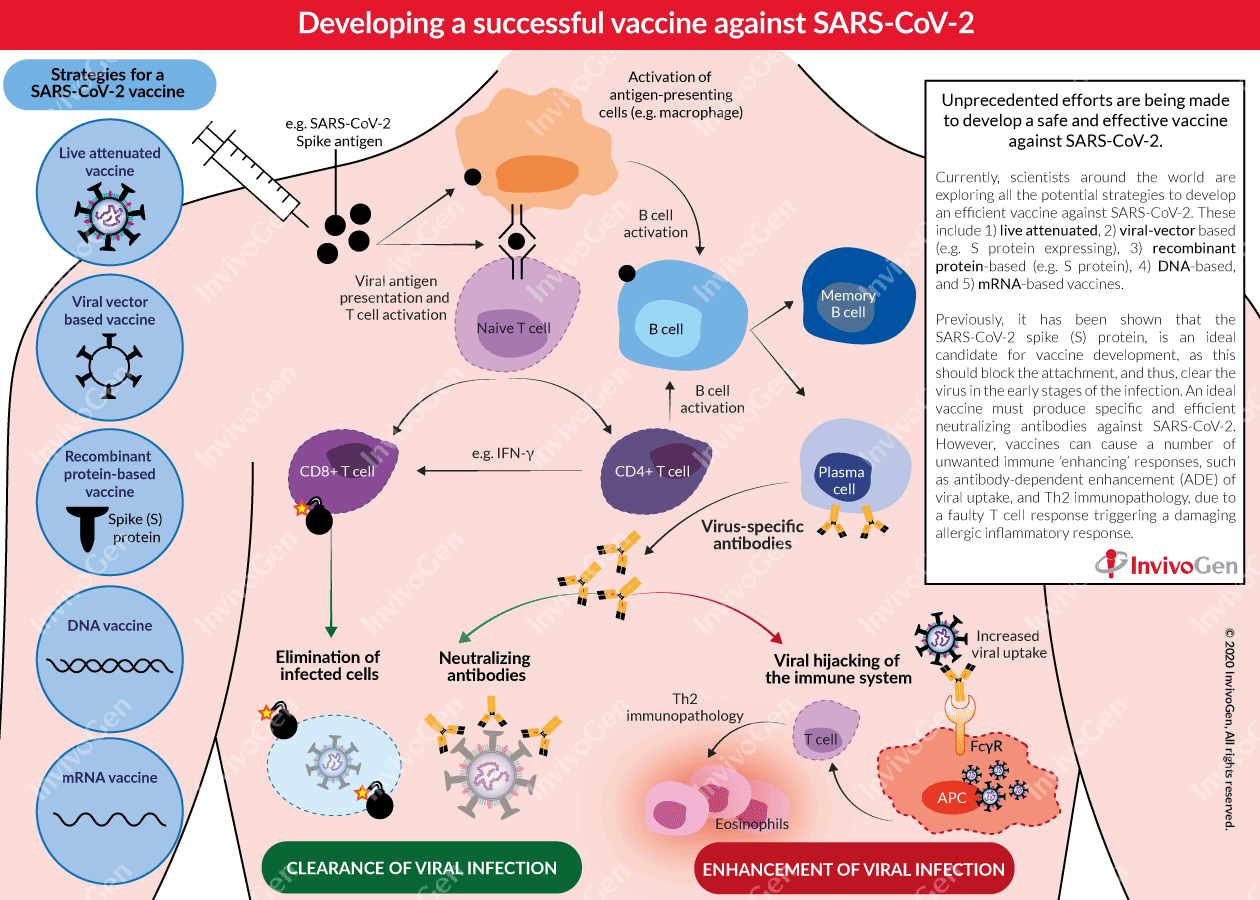
Developing a successful vaccine against SARS-CoV-2
The vaccine development effort in response to the COVID-19 pandemic is unprecedented in terms of both scale and speed. Importantly, SARS-CoV-2 vaccines will be essential in reducing morbidity and mortality if/when the virus establishes itself in the population. The race is now on for the development of both a safe and, effective vaccine against SARS-CoV-2.
Lessons learned from previous outbreaks
Previously, several vaccines for the closely related β-coronaviruses, SARS-CoV and MERS-CoV, have been developed and tested in animal models, including recombinant S-protein-based vaccines, attenuated and whole inactivated vaccines, and viral-vectored vaccines [1]. Most of these vaccines were found to protect animals from a challenge with SARS-CoV or MERS-CoV, although many did not induce long-term immunity. Additionally, in some cases, vaccination resulted in complications including lung damage and infiltration of eosinophils in a mouse model [2-4]. Despite viral differences, a lot can still be learned from these vaccines regarding how to move forward with the SARS-CoV-2 vaccine design.
Current strategies being explored for vaccine development
Currently, scientists around the world are exploring all the potential strategies to develop an efficient vaccine against SARS-CoV-2. Most vaccine candidates for COVID-19 aim to induce neutralizing antibodies against the viral spike (S) protein, preventing uptake via the human ACE2 receptor and thereby blocking infection [3, 5, 6]. A striking feature of this COVID-19 vaccine development landscape is the diversity of technology platforms being evaluated, including:
- Live attenuated vaccines: Use a modified ‘live’ SARS-CoV-2 virus with reduced virulence (e.g. codon deoptimization or a mutated E protein [7]). This strategy can induce a quick and strong immune response but can be dangerous for immunosuppressed persons.
- Viral-vector based vaccines: Use a viral backbone (e.g. adenovirus) to introduce a SARS-CoV-2 gene into the host. This strategy can enhance immunogenicity without an adjuvant and promotes a robust cytotoxic T cell response to eliminate virus-infected cells.
- Recombinant protein-based vaccines: Use SARS-CoV-2 proteins (e.g. S protein) to elicit an immune response in the host. Commonly used in combination with an adjuvant for improved immunogenicity.
- DNA vaccines: Use plasmid DNA to express antigens of SARS-CoV-2 for efficient delivery into the host cells. There are currently no approved DNA vaccines for humans.
- mRNA vaccines: Encodes a SARS-CoV-2 antigen and uses a system such as a liposome for delivery into the host. There are currently no approved mRNA vaccines for humans.
To date, the most advanced vaccine candidates for COVID-19 that have moved into human safety and efficacy clinical trials (I and II), include an mRNA-based vaccine developed by Moderna (NCT04283461) and an adenovirus type 5 viral vector-based vaccine developed by CanSino Biologicals (NCT04341389). Additionally, adjuvants such as AS03 and MF59 are being explored in recombinant S-protein based SARS-CoV-2 vaccines, to enhance immunogenicity and make lower doses possible, enabling vaccination of a wider population of people. A successful vaccine must produce both specific and, efficient neutralizing antibodies against SARS-CoV-2. Importantly, previous experience in the development of vaccines against SARS and MERS indicates the potential for damaging and unwanted immune enhancement effects. Therefore, careful and thorough testing is needed before the release of a global COVID-19 vaccine.
Major pitfalls in vaccine development
Vaccines can cause a number of adverse outcomes that are detrimental to the host due to unwanted immune ‘enhancing’ responses, including antibody-dependent enhancement (ADE) of viral uptake and Th2 immunopathology, due to a faulty T cell response triggering damaging allergic inflammation.
- ADE – antibody-dependent enhancement of viral infection
ADE is a process by which the virus leverages the antibodies to aid its infection. The immunopathological effects of ADE have been observed in various viral infections, characterized as antibody-mediated enhancement of viral entry and induction of a severe inflammatory response [8, 9]. ADE allows the infection of phagocytic antigen-presenting cells (APC), such as macrophages, due to the binding of virus-bound antibodies to FcγR on their surface [9]. Previous studies of MERS and SARS have brought attention to the possibility of ADE in COVID-19. It has been shown that neutralizing antibodies targeting the receptor-binding domains (RBD) of the MERS-CoV and SARS-CoV spike proteins, respectively, can mediate the entry of the viruses into Fc receptor-expressing human cells in vitro [10]. Furthermore, T cells, believed to play an important role in controlling COVID-19 infection, are depleted in severe COVID-19 disease [11], and this may be accelerated by APC infection due to ADE [12]. Recently, scientists have reported that unlike in MERS and SARS, the S-protein RBD of SARS-CoV-2 can elicit a robust neutralizing antibody response without inducing ADE in animal immunization studies [13].
Two studies (based on small cohorts of COVID-19 patients) have shown that an increased IgG response and a higher titer of total antibodies were associated with more severe disease [14, 15]. This is suggestive of possible ADE in SARS-CoV-2 infection [16]. However, some experts have doubts about the relevance of ADE in COVID-19.
- Th2 immunopathology
Some scientists believe that the symptoms of severe COVID-19 patients may be better explained by Th2 immunopathology, which is a dysregulated T cell response with a heightened response of virus-specific CD4+ T cells biased toward the Th2 subset causing allergic inflammation (e.g. IL-4, IL-5, and IL-13) and an influx of eosinophils into the lung [17]. In previous SARS-CoV vaccine development in vivo studies, it has been shown that infection subsequent to vaccination resulted in failure to control viral replication, enhanced clinical disease, and pathology characterized by skewed Th2 responses, inflammation, and eosinophilic influx [4, 17]. Interestingly, it has been noted that this pathology may be linked to antibodies specifically targeting the nucleocapsid (N) protein [4], with reduced pathology seen in S-protein vaccination studies [17].
Future directions
The quest for a vaccine for COVID-19 is an urgent problem. An effective vaccine will play a significant role in curbing the spread of SARS-CoV-2. However, scientists are only at the beginning, with more information needed regarding not only the virus but also the specific immune response it elicits in the host.
References
1. Roper, R.L. & Rehm, K.E. 2009. SARS vaccines: where are we? Expert Rev Vaccines 8, 887-898.
2. Agrawal, A.S. et al. 2016. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother 12, 2351-2356.
3. Amanat, F. & Krammer, F. 2020. SARS-CoV-2 Vaccines: Status Report. Immunity
4. Bolles, M. et al. 2011. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol 85, 12201-12215.
5. Prompetchara, E. et al. 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 38, 1-9.
6. Thanh Le, T. et al. 2020. The COVID-19 vaccine development landscape. Nat Rev Drug Discov
7. DeDiego, M.L. et al. 2007. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J Virol 81, 1701-1713.
8. Taylor, A. et al. 2015. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev 268, 340-364.
9. Smatti, M.K. et al. 2018. Viral-Induced Enhanced Disease Illness. Front Microbiol 9, 2991.
10. Wan, Y. et al. 2020. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J Virol 94
11. Qin, C. et al. 2020. Dysregulation of the immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis
12. Ricke, D. & Malone, R. 2020. Medical Countermeasures Analysis of 2019-nCoV and Vaccine Risks for Antibody-Dependent Enhancement (ADE).
13. Quinlan, B.D. et al. 2020. The SARS-CoV-2 Receptor-Binding Domain Elicits a Potent Neutralizing Response Without Antibody-Dependent Enhancement. Immunity D-20-00389. (under review)
14. Zhang, B. et al. 2020. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. medRxiv
15. Zhao, J. et al. 2020. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis
16. Cao, X. 2020. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol
17. Tseng, C.T. et al. 2012. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One 7, e35421.



