Anti-hPD1-Ni-hIgG1
-
Cat.code:
hpd1ni-mab1
- Documents
ABOUT
Anti-human PD-1 antibody - Human IgG1 (high effector functions)
Anti-hPD1-Ni-hIgG1 is a Nivolumab biosimilar with an IgG1 isotype. Nivolumab is a fully human monoclonal antibody (mAb) targeting the human programmed cell death 1 (PD-1) receptor. This therapeutic mAb binds and thereby, blocks the activation of the PD-1 receptor, resulting in the activation of T cells and cell-mediated immune responses. Nivolumab is FDA-approved for the treatment of melanoma and metastatic squamous non-small cell lung cancer (NSCLC).
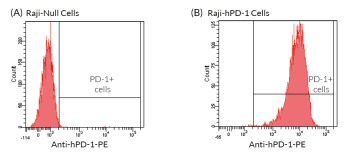
Anti-hPD1-Ni-hIgG1 comprises the variable region of Nivolumab and an IgG1 constant region for high effector functions (see figure).
This antibody can be used together with the cellular PD-1/PD-L1 assay (Bio-IC™) for screening and antagonist assays to disrupt the inhibitory interaction between PD-1 and PD-L1.
Key features
- Each lot is functionally tested and validated
- The complete sequence of the antibody construct has been verified
- Absence of endotoxins determined by the EndotoxDetect assay
All products are for research use only, and not for human or veterinary use.
SPECIFICATIONS
Specifications
PD-1
Human
Sodium phosphate buffer, glycine, saccharose, stabilizing agents
Negative (tested using EndotoxDetect™ assay)
Cellular assay, ELISA, flow cytometry, Fc interaction studies
Each lot is functionally tested and validated.
CONTENTS
Contents
-
Product:Anti-hPD1-Ni-hIgG1
-
Cat code:hpd1ni-mab1
-
Quantity:100 µg
Shipping & Storage
- Shipping method: Room temperature
- -20°C
- Avoid repeated freeze-thaw cycles
Storage:
Caution:
Details
Nivolumab background
Nivolumab is a fully human IgG4 (S228P) antibody that targets the programmed cell death 1 (PD-1) receptor found on activated T cells, B cells, and myeloid cells [1]. Under normal physiological conditions, PD-1 negatively regulates T cell activation, preventing autoimmunity. Under pathological conditions, cancer cells produce PD-L1 (programmed cell death 1 ligand 1), the agonist that binds and activates PD-1 [2]. Activated PD-1 enables cancer cells to evade the immune system. Nivolumab binds and inhibits the PD-1 receptor, thereby resulting in the activation of T cells. This therapeutic mAb contains an engineered hinge region mutation (S228P) designed to prevent the exchange of IgG4 molecules. Nivolumab is approved by the FDA and the EU for the treatment of a variety of cancer types, including non-small cell lung cancer, melanoma, and gastric cancer [3].
T Cell Activation
The activation of T lymphocytes initiates their proliferation and yields a variety of effector functions that allow for combating microbial infections, as well as developing tumors. The current paradigm is that full activation of T cells requires at least 2 signals upon contact with antigen-presenting cells (APCs) [4,5].
Signal 1 is delivered through the interaction of the T cell receptor (TCR) and a specific antigenic peptide associated with an MHC (major histocompatibility complex) molecule on APCs. Signal 2 is delivered through the interaction of CD28, the prototypical T cell co-stimulatory molecule, and its ligands, CD80 or CD86, expressed by the APC. However, several other molecules, named immune checkpoints (IC), have been reported to regulate the onset and the limitations of T cell activities. PD-1 (programmed cell death 1) receptor and its ligand, PD-L1, are among the best-characterized suppressive immune checkpoints [6].
Signal 1: TCR and [HLA::peptide]
The 'classical' and most represented TCR is an 80 to 90 kDa heterodimer composed of one α chain and one β chain. The αβTCR is a transmembrane protein expressed by developing and mature T cells. It features an extracellular ligand-binding pocket and a short cytoplasmic tail. Each αβTCR is restricted to a specific complex made of an antigenic peptide and a class I or class II MHC molecule. Human MHC molecules are also known as HLA (human leukocyte antigen). Because of its short cytoplasmic tail, the TCR, once engaged, lacks the ability to signal and requires non-covalent association with the CD3 to trigger downstream intracellular signaling and T cell activation [4, 5]. Importantly, signal 1 without co-stimulation results in T cell unresponsiveness or 'anergy', a tolerance mechanism that guards against premature activation.
Signal 2: CD28 and CD80/86
CD28 is a homodimeric and transmembrane protein expressed by T cells. Nearly all human CD4+ T cells and 50% of human CD8+ T cells express CD28. The CD28 interaction with CD80 (aka B7-1) or CD86 (aka B7-2) on APCs, in conjunction with TCR engagement, triggers a co-stimulation signal (signal 2). It results in T cell proliferation, cytokine production, cell survival, and cellular metabolism [4, 5].
Immune Checkpoint signal: PD-1 and PD-L1
— PD-1 (programmed cell death 1; also known as CD279) is a type I transmembrane protein expressed at the cell surface of activated and exhausted conventional T cells. PD-1 is an inhibitory immune checkpoint that prevents T-cell overstimulation and host damage. PD-1 interaction with its ligands PD-L1 and PD-L2 induces inhibition of TCR signaling [6].
— PD-L1 (programmed cell death ligand 1; also known as CD274 or B7-H1) is a transmembrane protein expressed at the cell surface of hematopoietic and nonhematopoietic cells and is induced by pro-inflammatory cytokines, such as in the tumor microenvironment [6]. PD-L1 is one ligand for PD-1, an inhibitory immune checkpoint receptor that is expressed by activated and exhausted T cells. PD-1:PD-L1 interaction induces inhibition of TCR signaling, thereby preventing T-cell overstimulation and host damage [6].
References:
1.McDermott D. & Atkins M., 2013. PD-1 as a potential target in cancer therapy. Cancer Med. 2: 662–73.
2. Tumeh PC. et al., 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 515(7528):568-71.
3. FDA website accessed in Dec 2024: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125554s112lbl.pdf
4. Budd R.C. & Fortner K.A., 2017. Chapter 12 - T Lymphocytes. Kelley and Firestein's Textbook of Rheumatology (Tenth Edition). pages 189-206.
5. Smith-Garvin J.E. et al., 2009. T Cell Activation. Ann. Rev. Immunol. 27:591-619.
6. Ribas A. and Wolchock J.D., 2018. Cancer immunotherapy using checkpoint blockade. Science. 359:1350-55.
DOCUMENTS
Documents
Technical Data Sheet
Safety Data Sheet
Certificate of analysis
Need a CoA ?