Murine Anti-mIL-16 mAb
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-mIL-16-mIgG1e3 Recombinant mouse mAb against mIL-16 (clone 14.1). For in vitro use. |
Show product |
200 µg |
mil16-mab15-02
|
|
||
|
Anti-mIL-16-mIgG1e3 InvivoFit™ Recombinant mouse mAb against mIL-16 (clone 14.1). For in vivo use. |
Show product |
1 mg |
mil16-mab15-1
|
|
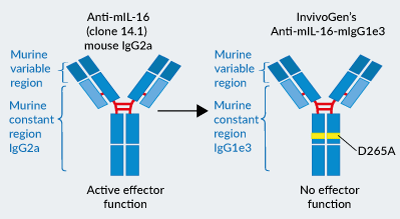
InvivoGen’s engineered Anti-mIL-16-mIgG1e3 antibody
 InvivoGen also offers:
InvivoGen also offers:
Recombinant mAb against murine IL-16
InvivoGen provides a recombinant Anti-mIL-16-hIgG1e3 monoclonal antibody (mAb) derived from the anti-IL-16 clone 14.1, originally produced in hybridoma [1]. Interleukin 16 (IL-16) is a pro-inflammatory cytokine playing an important role in modulating T cell activation, chemotaxis, and proliferation. It is associated with the development of several cancers as well as inflammatory diseases [2], which has prompted researchers to identify IL-16-targeting therapeutics [3].
Anti-IL-16 clone 14.1 crossreacts with mouse and human IL-16 [4, 5, and in-house data]. This neutralizing antibody inhibits murine and human T cell chemotaxis upon incubation with IL-16 in vitro [5] and reduces T cell-mediated renal injury in mice [6].
Anti-mIL-16-mIgG1e3 was engineered to feature the original mouse-derived variable regions [3] and an effectorless murine IgG1e3 constant region.
Anti-mIL-16-mIgG1e3 is produced in Chinese hamster ovary (CHO) cells, ensuring reliability and lot-to-lot reproducibility. Moreover, using mAb recombinant technology prevents common hybridoma-related drawbacks, such as the expression of non-relevant mAbs featuring aberrant light chains [7].
InvivoGen provides this antibody in two grades:
- In vitro use: Anti-mIL-16-mIgG1e3
- In vivo use: Anti-mIL-16-mIgG1e3 InvivoFit™
All InvivoFit™ products are handled in a clean room, filter-sterilized, and tested for bacterial contaminants. Additionally, this grade guarantees a low level of endotoxins (<1 EU/ml). The buffer formulation is specifically adapted for in vivo studies.
Key features:
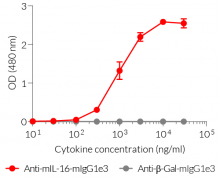
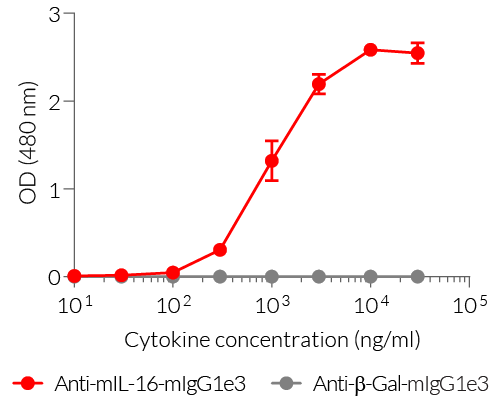
- Detection of mIL-16 (see figure)
- The antibody sequence is 100% murine
- Murine IgG1e3 isotype (constant region)
- mIgG1e3 (IgG1 with a replacement of aspartic (D) acid by alanine (A) at position 265 point mutation) is effectorless
- Free from non-relevant mAbs found in hybridoma-based productions
- Produced in animal-free facilities and defined media
- Low aggregation < 5%
- InvivoFit™ grade is available
References:
1. Theodore AC. et al. 1996. CD4 ligand IL-16 inhibits the mixed lymphocyte reaction. J Immunol, 157(5), 1958-64.
2. Amiel C, et al., 1999. Interleukin-16 (IL-16) inhibits human immunodeficiency virus replication in cells from infected subjects, and serum IL-16 levels drop with disease progression. J Infect Dis.;179(1):83-91.
3. Hall G. et al., 2016. Structure of a potential therapeutic antibody bound to interleukin-16 (IL-16): mechanistic insights and new therapeutic opportunities. J Biol Chem. 219(32):16840-8.
4. Hessel EM. et al., 1998. Involvement of IL-16 in the induction of airway hyper-responsiveness and up-regulation of IgE in a murine model of allergic asthma. J Immunol. 160(6):2998-3005.
5. Keane J. et al., 1998. Conservation of Structure and Function Between Human and Murine IL-16. The Journal of Immunology Vol. 160, Issue 12.
6. Wang S. et al., 2008. Decreased renal ischemia-reperfusion injury by anti-IL-16 inactivation. Kidney Int. 73(3):318-26.
7. Bradbury A. et al. 2018. When monoclonal antibodies are not monospecific: Hybridomas frequently express additional functional variable regions. mAbs, 10(4), 539–546.
Specifications
Target: Murine IL-16 (mIL-16)
Specificity: Cross-reactivity with human IL-16
Clone: 14.1
Source: CHO cells
Isotype: Murine IgG1e3 (D265A mutation; no effector function), kappa
Control: Murine IgG1e3 Isotype Control
Formulation of Anti-mIL-16-mIgG1e3: Lyophilized from 0.2 µm filtered solution in a sodium phosphate buffer with glycine, saccharose, and stabilizing agents.
Formulation of Anti-mIL-16-mIgG1e3 InvivoFit™: Lyophilized from 0.2 µm filtered solution in a sodium phosphate buffer and 5 % saccharose.
Tested applications: Detection (Western blot, ELISA)
Quality control:
- These products have been validated using ELISA and Western blot.
- The complete sequence of these antibodies has been verified.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells.
- The endotoxin level in Anti-mIL-16-mIgG1e3 InvivoFit™ is <1 EU/mg (determined by a LAL assay).
Contents
Please note: Each mAb is sold separately. See TDS for the exact contents.
Anti-mIL-16-mIgG1e3
- mil16-mab15-02: 200 µg, lyophilized
Anti-mIL-16-mIgG1e3 InvivoFit™
- mil16-mab15-1: 1 mg, lyophilized
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Store lyophilized antibody at -20 °C.
Store lyophilized antibody at -20 °C.
![]() Lyophilized product is stable for at least 1 year.
Lyophilized product is stable for at least 1 year.
![]() Avoid repeated freeze-thaw cycles.
Avoid repeated freeze-thaw cycles.
InvivoFit™
InvivoFit™ is a high-quality standard specifically adapted to in vivo studies. InvivoFit™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room. The level of bacterial contaminants (endotoxins and lipoproteins) in each lot is verified using a LAL assay and a TLR2 and TLR4 reporter assay.
Back to the topDetails
IL-16: a pleiotropic cytokine
Interleukin 16 (IL-16, initially named lymphocyte chemoattractant factor (LCF)) is a pro-inflammatory cytokine playing an important role in modulating T cell activation, chemotaxis, and proliferation [1]. It has also been classified as an alarmin, conveying an endogenous danger signal when released by stressed or necrotic cells [2]. IL-16 initially gained attention due to its activating functions in CD4+ T cells, as well as its contribution to inhibiting HIV replication [3]. It has since been also associated with the development of several cancers as well as the exacerbation of infectious, immune-mediated, and autoimmune inflammatory disorders [4, 5]. More recently, high plasmatic levels of IL-16 were found to correlate with COVID-19 severity [6, 7].
IL-16 production
IL-16 is produced by a variety of immune (e.g. T cells, eosinophils, neutrophils, dendritic cells) and non-immune (e.g. fibroblasts, epithelial, neuronal) cells [1, 8, 9]. It is synthesized as a large (~80 kDa) inactive precursor protein (pro-IL-16) stored inside the cell prior to activation [10]. Caspase-3 mediates the pro-IL-16 proteolytic cleavage and the release of two functional proteins. The cytokine function is exclusively attributed to the secreted C-terminal region (Cter-IL-16 or IL-16C, ~14 kDa), while the N-terminal product may play a role in cell cycle control [11, 12]. The exact stimuli that trigger pro-IL-16 processing by caspase-3 are not yet fully elucidated and differ with the cell type. Among human T lymphocytes, resting CD8+ T cells contain constitutively active caspase-3 and therefore, stored intra-cytoplasmic bioactive IL-16C. On the contrary, activation of caspase-3 in CD4+ T cells requires TCR stimulation [13]. In human neutrophils, pro-IL-16 is processed by caspase-3 when the cells undergo apoptosis [9]. In a model of human lung epithelial cells, SARS-CoV-2 infection triggers the release of bioactive IL-16C, possibly as a consequence of the NLRP1 inflammasome activation [7].
Bioactive IL-16C release
The precise secretion mechanism of mature IL-16 is still unclear. IL-16 amino acid sequence does not contain a secretory signal peptide, suggesting an unconventional secretion pathway. Multiple unconventional secretion pathways may be at play, depending on the cell type. In human neutrophils, bioactive IL-16C was shown to be released passively upon secondary necrosis [9, 10]. In lung epithelial cells, it may be released through Gasdermin E pores forming at the cell membrane in response to the NLRP1 inflammasome activation [7].
Bioactive IL-16C sensing and functions
IL-16 was initially described as a T cell-specific chemoattractant factor that mediates its functions upon binding to the CD4 transmembrane molecule [14], and more precisely to the D4 domain of CD4 [15]. Several lines of evidence indicate that IL-16C exerts its biological activity as homo-tetramers [10, 15, 16]. In CD4+ T cells, IL-16 triggers cell cycle progression and chemotaxis. The intracellular molecules thought to conduct the signal from the surface CD4 to the cytoskeleton for motility include PI3K (Phosphoinositide 3-kinase) and PLCγ (phospholipase C gamma) [17].
IL-16 sensing and signaling outcomes differ among cell types. Indeed, in a variety of myeloid cells (e.g. monocytes, macrophages, eosinophils, mast cells) which express CD4, this receptor seems to be dispensable for IL-16-mediated functions [18, 19]. Moreover, IL-16-stimulated monocytes, unlike T cells, produce pro-inflammatory cytokines, such as IL-6 and TNF-α [19].
Among the possible alternate receptors for IL-16, the tetraspanin CD9 surface molecule has been shown to participate in the IL-16-mediated chemotaxis and activation of mast cells [20]. Likewise, CD9 has been suggested as a receptor for IL-16 in human lung cells using the A549 cellular model [21]. Of note, IL-16 may also trigger chemotaxis indirectly, by inducing the expression of other chemokines. Indeed, IL-16 can act on lung epithelial cells to induce the upregulation of neutrophil-attracting chemokines, such as CXCL10 [22].
References:
1. Cruikshank, W.W., et al., 2000. Interleukin-16. J Leukoc Biol. 67(6): p. 757-66.
2. Rider, P., et al., 2017. Alarmins: Feel the Stress. The Journal of Immunology. 198(4): p. 1395-1402.
3. Amiel, C., et al., 1999. Interleukin-16 (IL-16) inhibits human immunodeficiency virus replication in cells from infected subjects, and serum IL-16 levels drop with disease progression. J Infect Dis. 179(1): p. 83-91.
4. Glass, W.G., et al., 2006. Not-so-sweet sixteen: the role of IL-16 in infectious and immune-mediated inflammatory diseases. J Interferon Cytokine Res. 26(8): p. 511-20.
5. Richmond, J., et al., 2014. Regulation of Cellular Processes by Interleukin-16 in Homeostasis and Cancer. Journal of Cellular Physiology. 229(2): p. 139-147.
6. Lucas, C., et al., 2020. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 584(7821): p. 463-469.
7. Planès, R., et al., 2022. Human NLRP1 is a sensor of pathogenic coronavirus 3CL proteases in lung epithelial cells. Molecular Cell. 82(13): p. 2385-2400.e9.
8. Wilson, K.C., et al., 2004. The effect of interleukin-16 and its precursor on T lymphocyte activation and growth. Growth Factors. 22(2): p. 97-104.
9. Roth, S., et al., 2015. Secondary necrotic neutrophils release interleukin-16C and macrophage migration inhibitory factor from stores in the cytosol. Cell Death Discov. 1: p. 15056.
10. Cruikshank, W.W., et al., 1994. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci U S A. 91(11): p. 5109-13.
11. Baier, M., et al., 1997. Molecular cloning, sequence, expression, and processing of the interleukin 16 precursor. Proc Natl Acad Sci U S A. 94(10): p. 5273-7.
12. Zhang, Y., et al., 1998. Processing and activation of pro-interleukin-16 by caspase-3. J Biol Chem. 273(2): p. 1144-9.
13. Wu, D.M., et al., 1999. Processing and release of IL-16 from CD4+ but not CD8+ T cells is activation dependent. J Immunol. 162(3): p. 1287-93.
14. Center, D.M., et al., 1996. Interleukin 16 and its function as a CD4 ligand. Immunology Today, 17(10): p. 476-481.
15. Liu, Y., et al., 1999. Identification of a CD4 domain required for interleukin-16 binding and lymphocyte activation. J Biol Chem. 274(33): p. 23387-95.
16. Keane, J., et al., 1998. Conservation of structure and function between human and murine IL-16. J Immunol. 160(12): p. 5945-54.
17. Cruikshank, W.W., et al., 1998. Signaling and Functional Properties of lnterleukin-16. International Reviews of Immunology. 16(5-6): p. 523-540.
18. Mathy, N.L., et al., 2000. Cutting edge: CD4 is not required for the functional activity of IL-16. J Immunol. 164(9): p. 4429-32.
19. Mathy, N.L., et al., 2000. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology. 100(1): p. 63-9.
20. Qi, J.C., et al., 2006. Human and mouse mast cells use the tetraspanin CD9 as an alternate interleukin-16 receptor. Blood. 107(1): p. 135-42.
21. Blake, D.J., et al., 2018. Ablation of the CD9 receptor in human lung cancer cells using CRISPR/Cas alters migration to chemoattractants including IL-16. Cytokine. 111: p. 567-570.
22. Smith, S., et al., 2018. IL-16/miR-125a axis controls neutrophil recruitment in pristane-induced lung inflammation. JCI Insight. 3(15).