Biosimilar Immune Checkpoint mAbs
The groundbreaking development of immune checkpoint (IC) blockade with the use of monoclonal antibodies (mAbs) against CTLA-4, PD-1, and PD-L1 has revolutionized cancer treatment [1]. There is now a focus on further developing these therapies by characterizing and targeting other co-inhibitory as well as co-stimulatory ICs [2,3].
The efficacy of antibodies is governed by their variable region conferring antigen specificity and their constant region triggering various effector functions, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP). It is well established that the effector functions, linked to the isotype, are key to ensure the therapeutic success of mAbs. One approach to enhance the efficiency of mAb-based therapeutics is to use engineered mAb isotypes by modifying their natural immunoglobulin (Ig) constant regions [3].
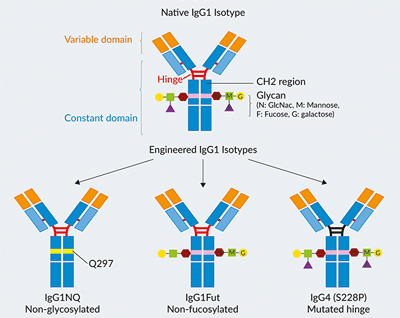
Examples of Strategies to Modify IgG mAb Isotypes
InvivoGen provides a growing collection of biosimilar mAbs to target ICs with various Ig isotypes. InvivoGen's biosimilar mAbs are for research use only products that are highly similar to their clinically approved counterpart.
Natural isotypes:
- IgG1 - induces potent ADCC, ADCP, and CDC
- IgG2 - induces poor ADCC and ADCP, while retaining some CDC function
- IgA2 - induces low ADCC and ADCP, and no CDC
Engineered isotypes (for modified effector functions):
- IgG1fut - induces increased ADCC due to defucosylation of the glycan sequences
- IgG1NQ and IgG1 (N298A) - induce no ADCC nor ADCP, and only minimal CDC due to mutations in glycosylation sites of the CH2 region
- IgG4 (S228P) - induces reduced ADCC, ADCP, and no CDC
InvivoGen's immune checkpoint mAb isotype families are useful tools in the study of effector functions in cancer immunotherapy, notably by choosing the most suitable isotype for your application.
InvivoGen’s biosimilar mAbs are generated by recombinant DNA technology, produced in CHO cells, and purified by affinity chromatography, ensuring batch-to-batch consistency. They are functionally validated by flow cytometry and sterile-filtered. All biosimilar mAbs feature the human kappa light chain.
References:
1. Wei, S.C. et al. 2018. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 8, 1069-1086.
2. Donini, C. et al. 2018. Next-generation immune-checkpoints for cancer therapy. J Thorac Dis 10, S1581-S1601.
3. Mazzarella, L. et al. 2019. The evolving landscape of 'next-generation' immune checkpoint inhibitors: A review. Eur J Cancer 117, 14-31.
4. Wang, X. et al. 2018. IgG Fc engineering to modulate antibody effector functions. Protein Cell 9, 63-73.




