Anti-CD27 (Varlilumab biosimilar) antibody family
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-hCD27-hIgG1 Human CD27 (Varlilumab) antibody - Human IgG1 |
Show product |
100 µg 3 x 100 µg |
hcd27-mab1
|
|
||
|
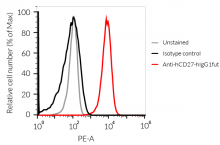
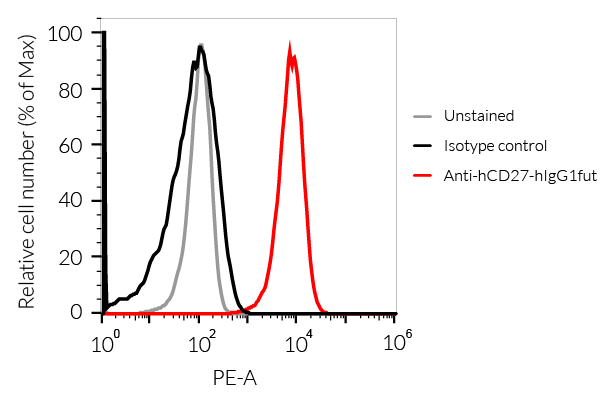
Anti-hCD27-hIgG1fut Human CD27 (Varlilumab) antibody - Human IgG1fut |
Show product |
100 µg 3 x 100 µg |
hcd27-mab13
|
|
||
|
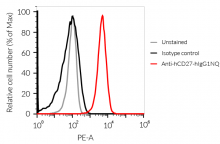
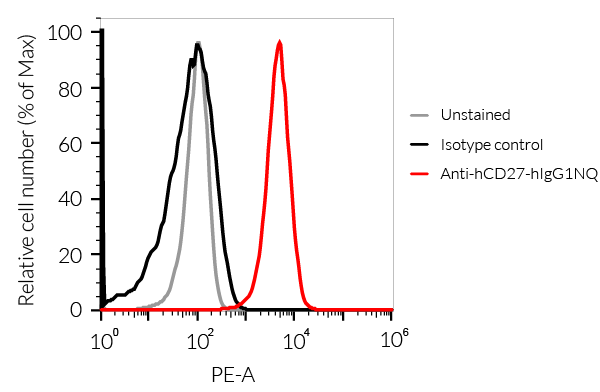
Anti-hCD27-hIgG1NQ Human CD27 (Varlilumab) antibody - Human IgG1fNQ |
Show product |
100 µg 3 x 100 µg |
hcd27-mab12
|
|
Human monoclonal antibody (mAb) isotypes against human CD27
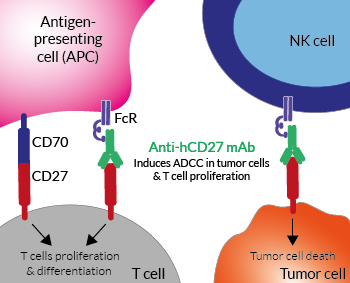
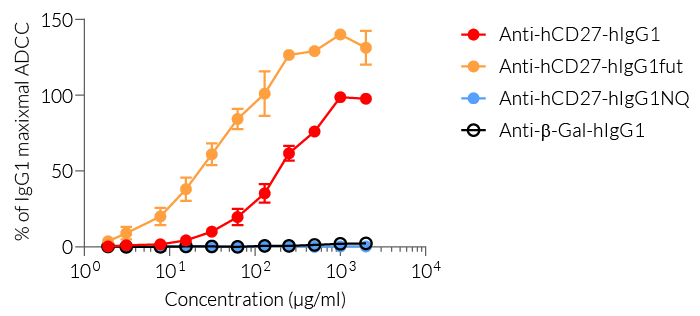
Anti-hCD27 mAb induces ADCC in cancer
(click to enlarge)
InvivoGen provides a family of anti-hCD27-derived monoclonal antibodies (mAbs) in multiple human isotypes.
They feature:
- the variable region of varlilumab targeting the human cluster of differentiation 27 (hCD27)
-
a constant region that mediates different effector functions depending on the isotype.
The effector functions of the human isotypes in this family include antibody‑dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). You may choose between:
— IgG1 for high effector functions ('wild-type' hIgG1 constant region),
— IgG1fut for even higher effector functions (engineered non-fucosylated (fut) constant region), or
— IgG1NQ for no effector functions (engineered N-glycosylation mutated constant region).
InvivoGen's mAbs have been generated by recombinant DNA technology, produced in Chinese hamster ovary (CHO) cells, and purified by affinity chromatography.
Anti-hCD27-hIgG1 is the biosimilar of the clinical antibody varlilumab. Varlilumab is a fully human agonist anti-CD27-hIgG1 mAb that activates the costimulatory molecule CD27 [1]. This receptor is constitutively expressed on the majority of mature T cells. In the appropriate context of T cell receptor engagement, the interaction of CD27 with its ligand CD70 (also known as CD27L) promotes T cell activation, maturation of effector capacity, and T cell memory [1-2]. This interaction became of interest to immunologists as a co-stimulatory immune checkpoint (IC) molecule and is the target of an anti-cancer drug in clinical trials [2].
Key features of the Anti-hCD27 isotype family:
- Clinically-relevant variable region targeting human CD27 (varlilumab)
- Choice of different constant regions for high/low effector functions
- Functionally validated by flow cytometry and ELISA
- The absence of bacterial contamination has been confirmed
InvivoGen’s products are for research use only, and not for clinical or veterinary use.
References:
1. Vitale LA, et al.,2012. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin Cancer Res.;18(14):3812-21.
2. Sanborn RE, et al., 2022 Safety, tolerability and efficacy of agonist anti-CD27 antibody (varlilumab) administered in combination with anti-PD-1 (nivolumab) in advanced solid tumors. J Immunother Cancer.;10(8):e005147.
Specifications
Specificity: Targets cells expressing human CD27
Clonality: Monoclonal antibodies
Clone: Varlilumab (Anti-hCD27-hIgG1, kappa)
Control: Human IgG1, hIgG1fut, hIG1NQ
Source: Chinese hamster ovary (CHO) cells
Formulation: 0.2 μm filtered solution in a sodium phosphate buffer with glycine, saccharose, and stabilizing agents.
Tested Applications: Flow cytometry, ELISA, ADCC assay
Purification:
- hIgG1: purified by affinity chromatography with protein G
- hIgG1fut: purified by affinity chromatography with protein G
- hIgG1NQ: purified by affinity chromatography with protein G
Quality control:
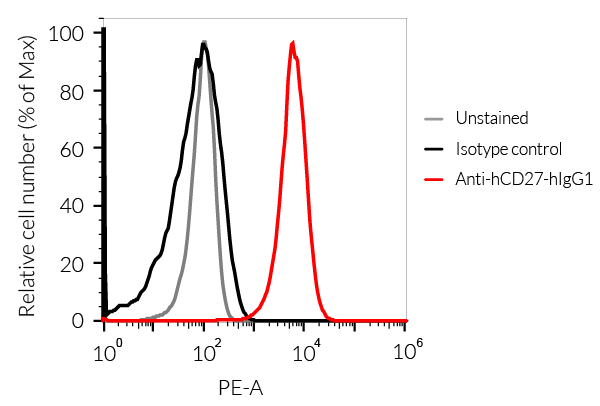
- The binding of Anti-hCD27 mAb to human CD27 on cells has been validated using flow cytometry.
- hIgG1 isotype effector function has been validated using an ADCC cellular assay.
- The complete sequence of the antibody has been verified.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ hTLR2 and HEK-Blue™ TLR4 cellular assays.
Contents
Please note: Each product is sold separately. These purified monoclonal antibodies are provided azide-free and lyophilized.
Anti-hCD27-hIgG1 is available in two quantities:
- hcd27-mab1: 100 µg
- hcd27-mab1-03: 3 x 100 µg
Anti-hCD27-hIgG1fut is available in two quantities:
- hcd27-mab13: 100 µg
- hcd27-mab13-03: 3 x 100 µg
Anti-hCD27-hIgG1NQ is available in two quantities:
- hcd27-mab12: 100 µg
- hcd27-mab12-03: 3 x 100 µg
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Upon receipt, store lyophilized antibody at -20 °C.
Upon receipt, store lyophilized antibody at -20 °C.
Tags: buy Varlilumab (Anti-TNFRSF7 / CD27) | Varlilumab (Anti-TNFRSF7 / CD27) supplier | purchase Varlilumab (Anti-TNFRSF7 / CD27) | Varlilumab (Anti-TNFRSF7 / CD27) cost | Varlilumab (Anti-TNFRSF7 / CD27) manufacturer | order Varlilumab (Anti-TNFRSF7 / CD27) | Varlilumab (Anti-TNFRSF7 / CD27) distributor
Back to the topDetails
The Cluster of Differentiation CD27, a member of the TNFR family, is known as the sole receptor for CD70 (aka CD27L). The CD27–CD70 costimulatory receptor-ligand pair plays an important role in immune regulation. In concert with the T cell receptor (TCR) crosslinking, it promotes T cell activation, proliferation, survival, maturation of effector capacity, and T cell memory [1-2]. In humans, CD27 is constitutively expressed on the majority of T cells, memory B cells and plasma cells, and natural killer (NK) cells. It is also expressed on various types of hematologic cancers. In leukemia, CD27 signaling leads to the induction of different pathways, supporting stemness, tumor cell proliferation, and self-renewal [3].
The efficacy of targeting the CD27/CD70 axis with an agonistic CD27 mAbs was shown in preclinical models of lymphoma, renal cell carcinoma (RCC), breast cancer, and sarcoma [4]. A human mAb directed at CD27 named varlilumab (also CDX-1127, 1F5) has entered clinical trials. It is able to activate CD27-positive T cells, while mediating the killing of CD27-expressing tumor cells [4-5]. Also, it has successfully completed a phase I/II dose escalation and cohort expansion study (NCT02335918) in combination with nivolumab in different solid malignancies. Further phase I trials with various combinations as well as phase II trials are still ongoing in renal cell carcinoma, squamous cell carcinoma of the head and neck, ovarian and colorectal cancers, and glioblastoma [4].
In conclusion, the CD27/70 axis is a promising pathway for immunotherapies showing a potential clinical benefit in different hematological and solid tumors. The combination of agonistic costimulatory CD27 mAbs with currently used immune checkpoint inhibitor-targeting mAbs (e.g. anti-PD-1) is foreseen to have a synergistic effect by different favorable effects on the tumor microenvironment. Further clinical trials are needed to investigate potential combinations of immune-modulating therapies, especially at earlier stages of disease, to achieve the best possible outcome for cancer patients [4].
References:
1. Jacobs, J. et al., 2015. CD70: An emerging target in cancer immunotherapy. Pharmacol Ther 155, 1-10.
2. Sanborn RE, et al., 2022 Safety, tolerability and efficacy of agonist anti-CD27 antibody (varlilumab) administered in combination with anti-PD-1 (nivolumab) in advanced solid tumors. J Immunother Cancer. 2022 Aug;10(8):e005147.
3. Flieswasser, T., Van den Eynde, A., Van Audenaerde, J. et al. 2022. The CD70-CD27 axis in oncology: the new kids on the block. J Exp Clin Cancer Res 41, 12.
4. Starzer AM, Berghoff AS., 2020. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open. 4 (Suppl 3):e000629.
5. Vitale LA, et al.,2012. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin Cancer Res. 2012 Jul 15;18(14):3812-21.