Anti-CTLA4 (Ipilimumab biosimilar - IgG1fut isotype)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-hCTLA4-hIgG1fut Human CTLA-4 (Ipilimumab) antibody - Human IgG1, non-fucosylated |
Show product |
100 µg 3 x 100 µg |
hctla4-mab13
|
|
Non-fucosylated human monoclonal antibody (mAb) against human CTLA-4
Anti-hCTLA4-hIgG1fut features the non-fucosylated (fut), constant region of the human IgG1 isotype and the variable region of ipilimumab. Ipilimumab is a fully human IgG1 monoclonal antibody that targets CTLA-4 (also known as CD152), a negative regulator of T cell activation. By binding CTLA-4, ipilimumab inhibits negative signals that physiologically downregulate T cell activation and exerts its therapeutic activity by upregulating the antitumor activity of T lymphocytes [1,2].
In addition, Ipilimumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) and TNF-α production [3]. Ipilimumab has been approved by the FDA for the treatment of unresectable or metastatic melanoma. Ipilimumab is undergoing clinical trials for other types of cancers, including lung cancer [4].
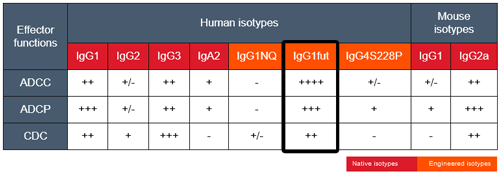
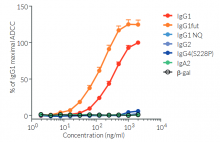
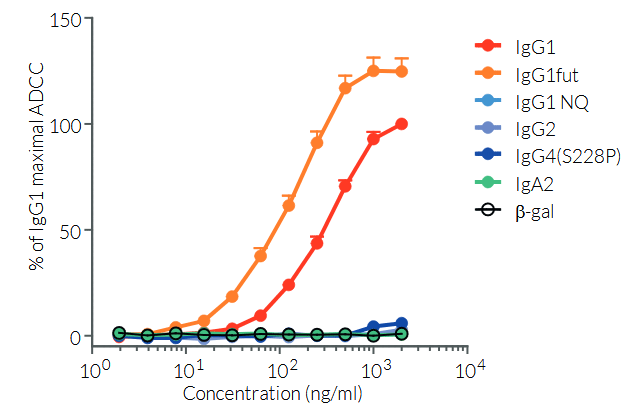
Anti-hCTLA4-hIgG1fut is a non-fucosylated antibody. The absence of the fucose residue from the N-glycans of IgG-Fc results in dramatic enhancement of antibody-dependent cellular cytotoxicity (ADCC) without any detectable change in complement-dependent cytotoxicity (CDC) or antigen-binding capability [5,6].
Anti-hCTLA4-hIgG1fut was generated by recombinant DNA technology. It has been produced in Chinese hamster ovary (CHO) cells deficient for fucosylation and purified by affinity chromatography with protein G.
More isotypes of this antibody are available and can be used for comparison of biological activities such as ADCC (see below or in the 'upon request' section).
References:
Grosso JF. & Jure-Kunkel MN., 2013. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 13:5.
Maio M. et al., 2013. Update on the role of ipilimumab in melanoma and first data on new combination therapies. Curr Opin Oncol. 25(2):166-72.
Laurent S.. et al., 2013. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med. 11:108.
Tomasini P., 2012. Ipilimumab: its potential in nonsmall cell lung cancer. Ther Adv Med Oncol. 4(2): 43–50.
Yamane-Ohnuki N. & Satoh M., 2009. Production of therapeutic antibodies with controlled fucosylation.corresponding MAbs. 1(3): 230–236.
Mizushima T., 2011. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells. 16(11): 1071–1080.
Specifications
Specificity: Targets cells expressing human CTLA-4
Clonality: Monoclonal antibody
Clone: Ipilimumab (Anti-hCTLA4-hIgG1, kappa)
Isotype: Human IgG1fut, kappa
Control: Human IgG1fut
Source: CHO cells
Purity: Purified by affinity chromatography with protein G
Formulation: 0.2 µm filtered solution in 68 mM sodium phosphate buffer (pH 7.4) with 91 mM glycine, 5% w/v saccharose, and stabilizing agents
Tested applications: Flow cytometry and ADCC
Quality control:
- Binding of Anti-hCTLA4-hIgG1fut to hCTLA4 has been validated using flow cytometry.
- The complete sequence of this antibody has been verified.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells.
Contents
Anti-hCTLA4-hIgG1fut purified monoclonal antibody is provided azide-free and lyophilized. It is available in two quantities:
- hctla4-mab13: 100 µg
- hctla4-mab13-03: 3 x 100 µg
![]() Product is shipped at room temperature.
Product is shipped at room temperature.
![]() Upon receipt, store at -20 °C.
Upon receipt, store at -20 °C.
Back to the top