Anti-PD-L1 (Atezolizumab biosimilar - IgG1fut isotype)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-hPD-L1-hIgG1fut Human PD-L1 (Atezolizumab) antibody - Human IgG1, non-fucosylated |
Show product |
100 µg 3 x 100 µg |
hpdl1-mab13
|
|
Non-fucosylated human IgG1 monoclonal antibody (mAb) against human PD-L1
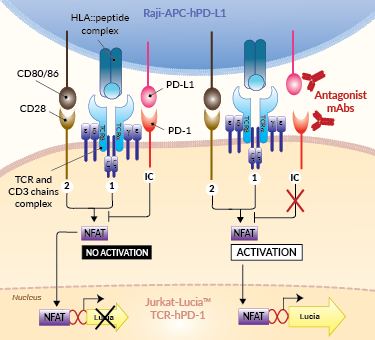
Principle of PD-1/PD-L1 cellular assay
(click to enlarge)
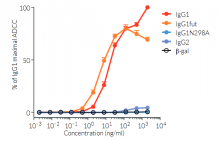
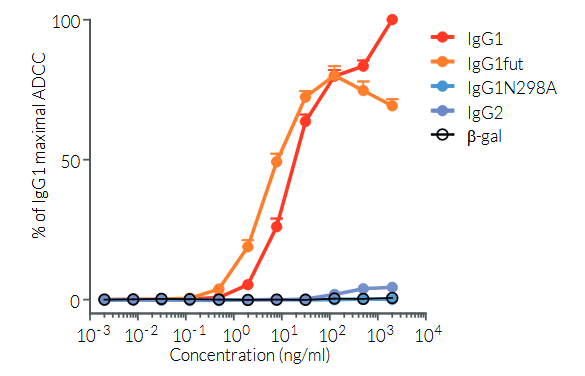
Anti-hPD-L1-hIgG1fut is a recombinant monoclonal antibody (mAb) featuring a variable region equivalent to atezolizumab, that recognizes human (h)PD-L1, and a non-fucosylated constant region of the human IgG1 (hIgG1fut) isotype. Anti-hPD-L1-hIgG1fut is a non-fucosylated antibody, whereby the absence of fucose residues from N-glycans of the IgG-Fc results in an enhancement of antibody-dependent cellular cytotoxicity (ADCC). This increased ADCC is without any detectable change to complement-dependent cytotoxicity (CDC) or antigen-binding capability. Anti-hPD-L1-hIgG1fut was generated by recombinant DNA technology, produced in CHO cells (deficient for fucosylation), and purified by affinity chromatography with protein G.
PD-L1 background
Programmed cell death ligand 1 (PD-L1), also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1) is a transmembrane protein that can be constitutively expressed or induced in myeloid, lymphoid, and normal epithelial cells, as well as in cancer [1, 2]. PD-L1 is the principal ligand for programmed cell death protein 1 (PD-1) and under physiological conditions, this interaction is essential in the development of immune tolerance preventing excessive immune cell activity. However, PD-L1 expression is an immune evasion mechanism exploited by various malignancies and is generally associated with poorer prognosis [3]. Specifically, over-expressed PD-L1 on tumor cells and tumor-infiltrating immune cells, such as macrophages, can bind to PD-1 on cytotoxic T cells, and ultimately inhibit the anti-tumor T cell response [2, 4]. Thus, due to PD-L1’s instrumental role in immune evasion by cancer cells, there are numerous inhibitors in development as promising immuno-oncology therapies. Notably, Atezolizumab (also known as MPDL3280A), a fully humanized IgG1 (N298A) mAb that blocks the interaction of PD-L1 with PD-1 and induces anti-tumor immune reactivation, has been approved by the FDA for combinational use in the treatment of lung and breast cancer [2, 5].
Key features of Anti-hPD-L1-hIgG1fut:
- Clinically relevant variable region targeting PD-L1
- Features the engineered hIgG1fut constant region for enhanced effector function (i.e. ADCC)
- Functionally validated by flow cytometry
The terms “Atezolizumab” and “MPDL3280A” are only used as references. Anti-hPD-L1-hIgG1fut is not a pharmaceutical biosimilar of Atezolizumab. It has not been developed nor approved by Atezolizumab owner(s), and is not intended for any therapeutic or diagnostic use in humans or animals.
References
1. Juneja, V.R. et al. 2017. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 214, 895-904.
2. Kythreotou, A. et al. 2018. PD-L1. J Clin Pathol 71, 189-194.
3. Sun, C. et al. 2018. Regulation and Function of the PD-L1 Checkpoint. Immunity 48, 434-452.
4. Lau, J. et al. 2017. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun 8, 14572.
5. Heimes, A.S. & Schmidt, M. 2019. Atezolizumab for the treatment of triple-negative breast cancer. Expert Opin Investig Drugs 28, 1-5.
Specifications
Specificity: Targets cells expressing human or murine PD-L1
Clonality: Monoclonal antibody
Clone: Atezolizumab (anti-hPD-L1-hIgG1 (N298A), kappa)
Isotype: Human IgG1fut, kappa
Control: Human IgG1fut
Source: CHO cells
Formulation: 0.2 µm filtered solution in 68 mM sodium phosphate buffer (pH 7.4) with 91 mM glycine, 5% w/v saccharose and stabilizing agents
Purification: Purified by affinity chromatography with protein G
Tested applications: Flow cytometry, ADCC, Disruption of PD-1/PD-L1 inhibitory interaction
Quality control:
- Binding of Anti-hPD-L1-hIgG1fut to hPD-L1 has been validated using flow cytometry.
- ADCC has been tested using CD16-expressing Jurkat-NFAT reporter cells.
- The complete sequence of this antibody has been verified.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells.
Contents
Anti-hPD-L1-hIgG1fut purified monoclonal antibody is provided azide-free and lyophilized. It is available in two quantities:
- hpdl1-mab13: 100 µg
- hpdl1-mab13-03: 3 x 100 µg
![]() Product is shipped at room temperature.
Product is shipped at room temperature.
![]() Upon receipt, store at -20 °C.
Upon receipt, store at -20 °C.
Back to the top